Team:Stanford/Research
From 2010.igem.org

| Home | Project | Applications | Modeling | Parts | Team | Notebook |
Contents |
Why Ratios?
Ratios rule the biological world, and controlling them will unlock a vast range of applications for synthetic biology.
In electrical and mechanical engineering, computers can be used to calculate a ratio from precise measurements, but biological engineering doesn't have that luxury. We need a hardwired device to compute and act on ratios independent of a controlling computer or human researcher.
The Stanford team's ratio sensors use biological machinery to eliminate the human component from ratio computation. Our output system is more efficient than two individual sensors and, most importantly, can pass its results on to other parts and devices. A ratio sensor performs a calculation that a user doesn't need to/won't be able to. A ratio sensor frees up a wavelength channel on the measurement device, and, more importantly for the future, allows the computed ratio to be passed on to another part. A useful ratio sensor that could be utilized in a broad regime of biological applications needs to be operable and accurate in micro-environments; the Stanford team's ratio sensor is engineered to function within E. Coli, which is fully operable over a range of environments.
Our Project: Two Designs for Ratio Detection
Our team decided to pursue two different systems for detecting ratios. After considering the scenarios in which biological engineers would want to use ratio sensors, having two specialized sensors seemed to allow greater flexibility of usage than a single design.
While both systems receive two input signals, the output they give is different, allowing them be applied in different situations. Here's a brief rundown of the two designs (you can see more by clicking through to the individual project pages).
Our First Sensor: The sRNA Sensor
Method: Small RNA Interference
Output: One of two possible proteins, depending on whether the ratio of input chemicals lies above or below a predetermined threshold
Useful: For reporting on a situation with a boolean output: disease detection, preterm labor warning, cancer metastasis warning
Data: Initial data gathered using FACS on part K353002, along with a more detailed description of our sRNA interference design can be found below.
Overview
Our first sensor design is tuned to a single ratio of input chemicals and tells the user if the input chemicals are present in relative concentrations below, at, or above that ratio.
L-arabinose binds to pBAD (BBa_I0500) which promotes the transcription of sRNA2, RSID1, and GFP. AHL binds to pLUX (BBa_F2620), which promotes the transcription of sRNA1, RSID2, and RFP. In the cytoplasm, each sRNA binds to its respective RSID before translation occurs. Ribosomes are prevented from binding to those mRNA's that are bound by sRNA's, meaning that only those mRNA's that remain unbound by sRNA's get translated into reporter protein. sRNA/mRNA dimers are eventually degraded by the cell's machinery.
If the concentration of L-arabinose is greater than that of AHL, more sRNA2 and GFP will be produced than sRNA1 and RFP. This means that each sRNA1 will be able to bind to a copy of RSID1, while there will not be enough sRNA2 to inhibit all of the RFP. An excess of L-arabinose therefore leads to an expression of GFP.
To change the threshold ratio detected by the sensor, multiple copies of the genes encoding either the mRNA or the sRNA can be placed downstream of the promoters.
By sensing only one ratio, we gain a sharp transition between no signal and a lot of signal. This allows us to insulate our output from biological noise in all regimes of the input domain except for the small neighborhood surrounding the sharp transition.
By engineering readout redundancy into the sRNA sensor, we have insulated our sensor from detector-specific readout noise. If there were only one color, we would register the transition from off -> on whenever the signal exceeded the signal/noise ratio of our detector. This means that we would actually read the off/on transition as occurring at different points in the input domain with different detectors! But with two colors, (one transitioning from off -> on, the other from on -> off), we can set the detection threshold far above our detector's S/N threshold, and average the transition point in the two channels to achieve a detector-independent measurement of the specified ratio.
Read about our other sensor or return to our project page.
The RSID: Improving sRNA Interference
Our system uses sRNA interference, which is usually a non-modular operation. Because each sRNA must be complementary to the mRNA sequence of the protein it inactivates, changing outputs in a traditional sRNA interference system would require designing and inserting a new sRNA for each output. To overcome this design flaw and make our sensor more easily modified, we implemented a novel control system for sRNA interference: the RSID.
The RSID is a synthetically-created DNA sequence that includes a ribosome binding site, and is inserted upstream of the output proteins. Each sRNA recognizes and binds to a specific RSID, covering the RBS contained within it and preventing the translation of the downstream output protein.
Because the RSID's are independent from the output protein, new output proteins may be inserted without affecting the performance or design of the internal mechanism. This feature allows our system to be modified by future users to release any necessary output protein.
In choosing the sequence of our RSID's and sRNA's, we were careful to avoid several possible pitfalls. Our sRNA's and RSID's are all non-complementary to anything in the host cell's genome in order to avoid accidentally inhibiting important cell processes. In addition, we screened our designs to make sure they had low levels of self-dimerization, which would limit their effectiveness.
Our RSID's are valuable parts in their own right, as they can be applied to any other projects employing sRNA interference to yield similar modularity and precision targeting.
Device and Parts Level Diagrams
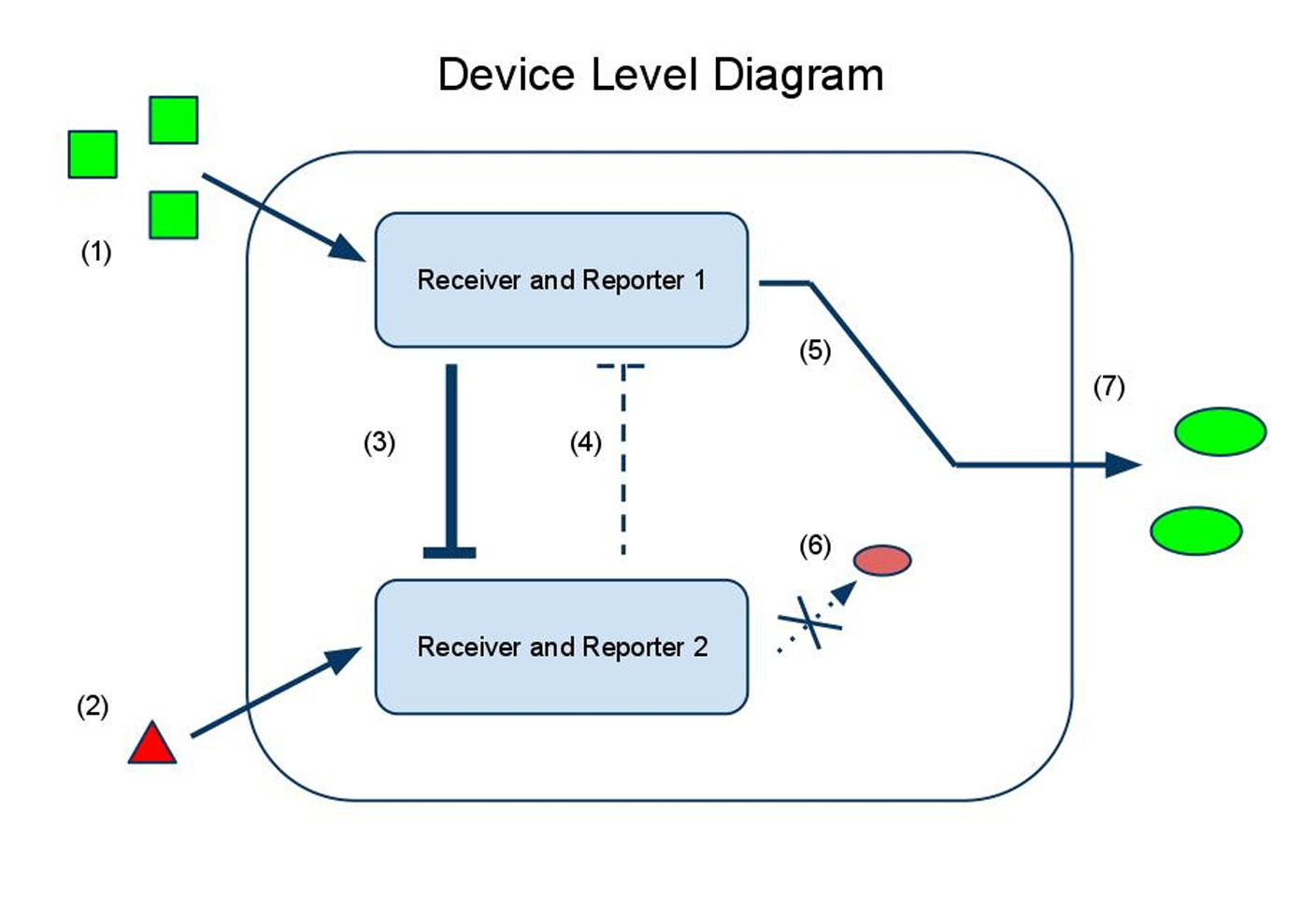
(1) Input A activates Receiver and Reporter 1. (2) Input B activates Receiver and Reporter 2. (3) There is more input A, so Receiver and Reporter 1 will strongly repress Receiver and Reporter 2. (4) There is less input B, so the repression going the other way is weaker. (5) This leads to the production of output A, and (6) little to no production of output B. (7) The output of the device is either dominated by output A or output B. By seeing which is the case, we can infer whether input A or input B was present in greater amounts.
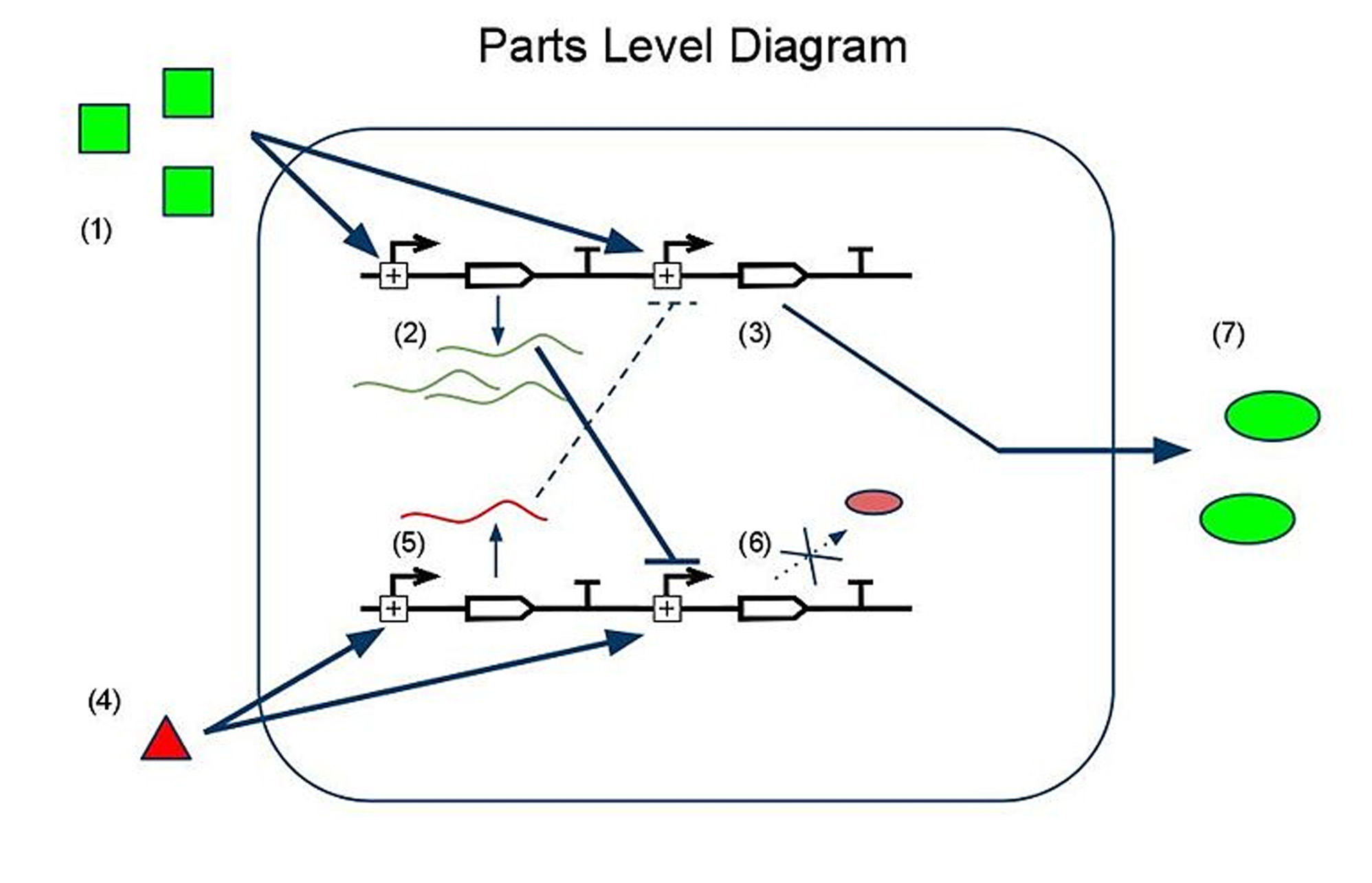
(1) Input A leads to the transcription of (2) GFP mRNA and (3) sRNA targeting RFP mRNA. This is how we implement both reporting and repressing outputs as a result of sengins input A. (4) Input B leads to two similar transcription events: (5) RFP mRNA and (6) sRNA targeting GFP mRNA. In this scenario, because there is more input A than input B, the GFP mRNA is translated whereas the RFP mRNA is silenced by its respective sRNA, thus leading to (7) a device output that is clearly more green than red, reflecting the relative levels of input A to input B.
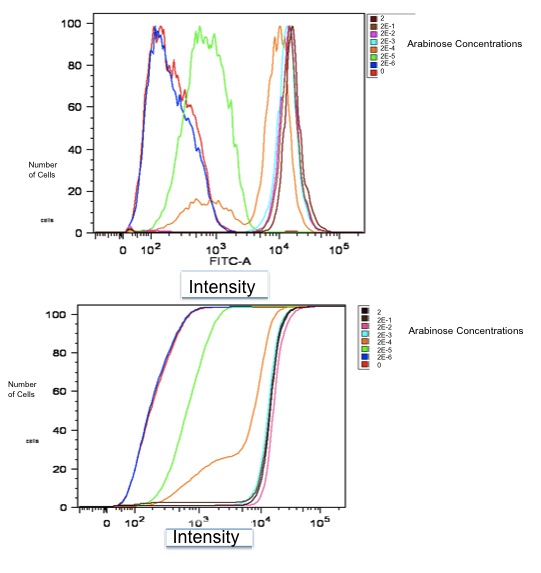
Flow Cytometry Data
Both of these graphs plot the output of part K353002 in BW27783 cells induced with a series of concentrations of L-arabinose, measured in molarity. The first is a histogram showing the distribution of expression levels at each concentration of L-arabinose. The second is the same data displayed as a cumulative distribution so that population medians are more apparent.
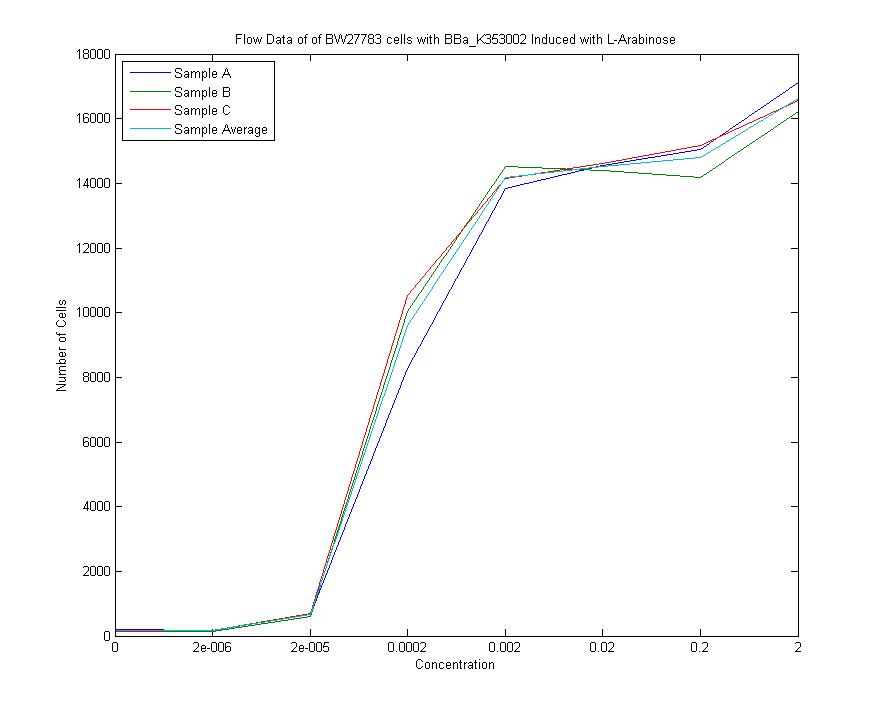
We measured a dose-response-curve of median output vs L-arabinose concentration in triplicate. This curve constitutes the basic characterization of part K353002, including measurement of the concentration of L-arabinose which gives half-maximal output, the steepness of transition from off to on, and the parts dynamic range. Fitting a Hill curve provided the following values plus or minus 95% confidence intervals:
- Half-max induction: 143 +- 36 uM
- Hill coefficient: 1.4 +- .6
- Fold Induction: 860
The Second Design:
Method: Kinase/Phosphatase Regulation of a Transcription Factor
Output: One output protein whose concentration is linearly dependent on the ratio of the input chemicals
Useful: For acting in a situation requiring a graded response: drug delivery, metabolic flux control, detailed ratiometric reporting
Data: Please refer to the following pages for data:
- http://partsregistry.org/Part:BBa_K353005
- http://partsregistry.org/Part:BBa_K353006
- http://partsregistry.org/Part:BBa_K353007
- http://partsregistry.org/Part:BBa_K353008
Both sensors are:
- Modular: input and output molecules can be changed without affecting the interior mechanism of the device
- Orthogonal: device mechanisms are not found in E. coli, avoid crosstalk with host cell
Biosafety
1. Would any of your project ideas raise safety issues in terms of researcher safety, public safety, or environmental safety?
Answer: There are no major safety risks regarding our general system design. In its current form, our device is only meant for ratiometric analysis of compounds within E. coli. As with any other biological hazard, typical laboratory precaution should be taken when handling cells resistant against antibiotics.
2. Is there a local biosafety group, committee, or review board at your institution?
Answer: There is a local board at Stanford University called Health and Safety at the Stanford University School of Medicine.
3. What does your local biosafety group think about your project?
Answer: We have not communicated with our local biosafety group regarding the design or safety of our project.
4. Do any of the new BioBrick parts that you made this year raise any safety issues?
Answer: Although the kinases and phosphatases chosen for our project come from Mycobacterium tuberculosis and Streptomyces coelicolor A3(2), our team advisors and graduate mentors believe that these proteins do not pose any significant safety risks.
Research
Medal Requirements
Bronze Medal
- Register the team, have a great summer, and have fun attending the Jamboree.
- Done!
- Successfully complete and submit a Project Summary form.
- Done!
- Create and share a Description of the team's project via the iGEM wiki
- Present a Poster and Talk at the iGEM Jamboree
- Booked our plane tickets!
- Enter information detailing at least one new standard BioBrick Part or Device in the Registry of Parts
- [http://partsregistry.org/wiki/index.php?title=Part:BBa_K353002 Done!]
- Entered information for each new part or device should at least include primary nucleic acid sequence, description of function, authorship, any relevant safety notes, and an acknowledgement of sources and references.
- [http://partsregistry.org/wiki/index.php?title=Part:BBa_K353002 Done!]
- Submit DNA for at least one new BioBrick Part or Device to the Registry of Parts.
- Done!
Silver Medal
- Demonstrate that at least one new BioBrick Part or Device of your own design and construction works as expected.
- Characterize the operation of at least one new BioBrick Part or Device and enter this information on the Parts or Device page via the Registry of Parts
Gold Medal
- Characterize or improve an existing BioBrick Part or Device and enter this information back on the Registry.
- When sequencing one of our ligations, we noticed an unexpected sequence in our results. After more investigation, we determined that that sequence came from the Distribution part [http://partsregistry.org/wiki/index.php?title=Part:BBa_E1010 BBa_E1010], and that the part sequence listed on the Parts Registry was incomplete. We have notified the Registry and made a note of this discovery on the E1010 parts experience page.
- Outlined and detailed a new approach to an issue of Human Practice in synthetic biology as it relates to your project, such as safety, security, ethics, or ownership, sharing, and innovation.
- With our Twitter project, we hope to help all iGEM teams by facilitating collaboration (i.e. sharing of data and inducing innovation) between teams. Read more about it here!
 "
"