Team:Slovenia/PROJECT/oscillator/exp
From 2010.igem.org
Contents |
Selection of versatile genetic oscillator building blocks
It hass been previously demonstrated that besides in prokaryotic systems T7 RNA polymerase is functional also in mammalian cells (Stein and Moss, 1990, Slovenian iGEM team 2007). It is also highly processive and selective for the specific T7 promoter. As such T7 RNA polymerase seemed an ideal driving force for orthogonal oscillators. When selecting repressor proteins for our system we soon realized that there are not enough natural repressors with matched properties, which would enable testing repressilators with at least five repressors in the cycle. Therefore we explored the use synthetic DNA-binding proteins. Synthetic DNA binding proteins of same type should have similar affinity, folding, stability and degradation properties. Matching of those properties is essential for sustained oscillations.
At the end we decided to investigate the function of TAL repressor for Smollen oscillator and zinc fingers for repressilator. Repressor based on TAL effector was designed de novo and zinc fingers were selected from the literature. There is also a database on the internet with more than 700 experimentally tested [http://bindr.gdcb.iastate.edu:8080/ZiFDB/ zinc finger domains]. When selecting zinc fingers we tried to avoid those, which would interfere with the normal cellular functions. All selected zinc fingers have high affinity for predicted DNA site and on the other hand do not bind to similar sequences. Also check our page about selecting DNA binding proteins.
Another question was if it is possible to repress transcription from the T7 promoter. It was previously shown that T7 promoter can be repressed by binding of Lac repressor to the target sequence as far as 18 nucleotides downstream of T7 promoter (Lopez et al., 1998). We used same principle but incorporated TAL or zinc finger binding sequence downstream of the promoter instead of Lac operator. This approach provides practically unlimited number of promoters for with matched characteristics for driving expression of oscillator components. Our next milestone was to test the function of artificial repressors in the biological system.
Cloning strategy
For Smolen type of oscillator (nicknamed NICilator) we fused the Tet operator downstream of T7 promoter. TAL repressor was designed in a way to bind to the Tet operator This selection provided the possibility to use the natural Tet repressor in the NICillator as the positive control. Venus yellow fluorescent protein was used as a reporter, because of its short maturation time. To enhance degradation speed of the reporter CL1 and PEST sequences were tagged to its C-terminal end. This was intended to prevent excessive accumulation of fluorescent reporter which would prevent online measurement of oscillations. In order to examine how the stability of different components influences the oscillation period, we also made several combinations of repressor protein and T7 RNA polymerase with degradation tags. Small amount of T7 RNA polymerase under the control of constitutive cytomegalovirus promoter was included into the system to ensure the start of oscillations and their maintenance. Transcriptional terminator was placed at the end of all constructs.
Similar constructs were made also for the repressilator. But here we used 3 different zinc finger proteins instead of TAL or Tet repressor. The corresponding zinc finger binding sequence was fused downstream of T7 promoter driving the expression of repressor in the next oscillating cycle. PEST and ssrA degradation tags were added at the C-terminal end of all zinc fingers. PEST sequence ensured fast degradation speed in eukaryotic system and ssrA sequence ensured fast degradation speed in prokaryotic system. Again Venus reporter protein was used as an a reporter of oscillations. Expression of reporter was driven from the T7 promoter, which was located upstream of DNA-binding sequence for one of the zinc fingers. To prevent the excessive accumulation of fluorescent reporter PEST (eukaryotic system) and ssrA (prokaryotic system) sequences were added to its C-terminal end. Small amount of T7 RNA polymerase under the control of constitutive cytomegalovirus promoter was included in system to ensure start of oscillations and their maintenance. Transcriptional terminator was placed at the end of all constructs.
Results
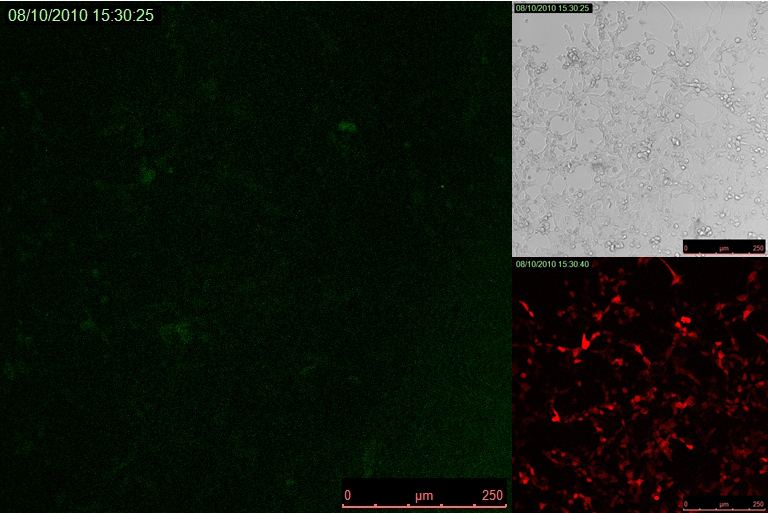
We observed efficient transcriptional repression of Venus reporter protein when TAL or TetR repressors were cotransfected with modified T7 promoter driving expression of fluorescent reporter. As can bee seen in Figure 3 cells transfected with fluorescent protein under the control of T7 promoter with TAL/TetR binding sequence immediately downstream of it become fluorescent. But almost complete repression of fluorescence occurs in cells cotransfected with either TAL repressor (Figure 4) or TetR (Figure 5) under CMV promoter. HEK 293 cells were excited with 514 nm Argon laser, emission of Venus fluorescent reporter was collected between 520 and 580 nm. Test of transfection efficiency was done by cotransfecting cells with red fluorescent protein mCherry. Red fluorescent protein was excited with 543 nm HeNe laser, its emission was collected between 580 and 650 nm.
We demonstrated that synthetically designed TAL effector-based DNA-binding protein efficiently repressed expression from the modified T7 promoter. Results prove that synthetic repressor, which can be prepared in numerous variants exhibits the required functionality required for creating genetic oscillators.With synthetic repressor proteins we will be able to design universal, orthogonal, robust and tunable types of oscillators in many different variants. This result represents the absec building block to test the results of modeling genetic oscillators. Unfortunately the time for the iGEM project did not allow us to test the functionality of assembled oscillators. Besides satisfying pure academic curiosity, there are many potential uses for synthetic oscillators such as drug delivery or information processing.
Lopez P.J., Guillerez J., Sousa R. and Dreyfus M. 1998. On the Mechanism of Inhibition of Phage T7 RNA Polymerase by lac Repressor. Journal of Molecular Biology, 276: 861-875.
Elroy-Stein O. and Moss B. 1990. Cytoplasmic expression system based on constitutive synthesis of bacteriophage T7 RNA polymerase in mammalian cells. Proceedings of the National Academy of Sciences of the United States of America, 87: 6743-6747.
 "
"