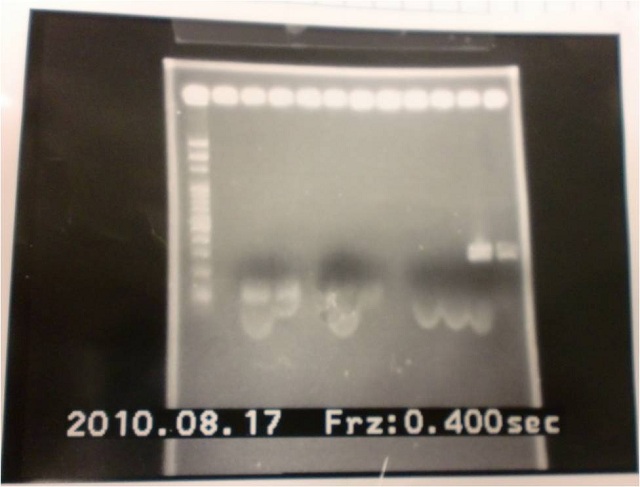
 |
| Descr | Win length | Fail length
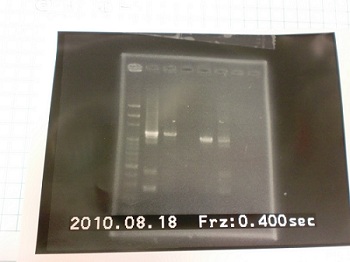
|
| 1: marker 100bp | |
|
| 2: ribo+vector | 292bp |
|
| 3: ribo+vector | 292bp |
|
| 4: - | - | -
|
| 5: Positive Control | 1100 bp | none
|
| 6: Negative Control | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| Total:(*2) | 50μl
|
| template | 2μl
|
| VF+VR | 2μl
|
| dNTP | 2μl
|
| Buffer | 5μl
|
| ddH2O | 39.75μl
|
| Enzyme tag | 0.25μl
|
|
- Cut gel 16:30
- Plasmid extraction 16:30
2010.08.23
- the upper experiment are failed,so we restart the experiment.
- Run gel 11:00
- After nanodrop, we found that the concentration is too low(only 3.0), so we re-PCR-of-primer again.
- PCR of primer 16:00
- Transformation(GFP) 17:15
2010.08.24
 |
| Descr | Win length | Fail length
|
| 1: | |
|
| 2: marker 100bp | |
|
| 3: | |
|
| 4: pcr riboswitch | 56bp |
|
| 5: | |
|
| 6: Positive Control | | 300bp
|
| 7: Negative Control | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| Total:(*2) | 50μl
|
| template | 2μl
|
| VF+VR | 2μl
|
| dNTP | 2μl
|
| Buffer | 5μl
|
| ddH2O | 39.75μl
|
| Enzyme tag | 0.25μl
|
|
- PCR Purify 10:45(45ul)
- Digest 11:15
| Total: | 30μl
|
| DNA | 26μl
|
| Digestion buffer | 2μl
|
| Enzyme 1(X) | 1μl
|
| Enzyme 2(P) | 1μl
|
- Digest purify 13:30
- Nanodrop(19.8ng/ul)
- Ligation 15:44
- Ligate riboswitch+pSB1A2(1:3) and ligate GFP+pSB1A2(1:2)(we use 2009's GFP+terminater so we have to ligate pSB1A2)
| Total: | 10μl
|
| DNA of vector | 1μl
|
| DNA of insert | 3μl
|
| buffer | 2μl
|
| ligase | 0.5μl
|
| ddH2O | 4.5μl
|
| Total: | 10μl
|
| DNA of vector | 1μl
|
| DNA of insert | 2μl
|
| buffer | 2μl
|
| ligase | 0.5μl
|
| ddH2O | 5.5μl
|
2010.08.25
- 3-in-1:GFP(1 colony),Riboswitch(2 colony) 17:00pm
- Run PCR 17:20pm
2010.08.26
- Run gel 10:30am
- Because we thought the result is unsure,we decide to digest to check.
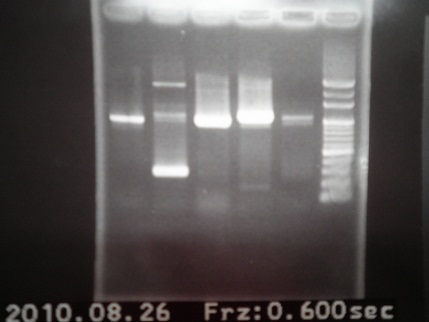 |
| Descr | Win length | Fail length
|
| 1: GFP | |
|
| 2: (1)riboswitch+pSB1A2 | 292bp |
|
| 3: (2)riboswitch+pSB1A2 | 292bp |
|
| 4: Positive Control | 1100bp |
|
| 5: Negative Control | Contamination~1000 bp |
|
| 6: Marker:100bp | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| Total:(*2) | 50μl
|
| template | 2μl
|
| VF+VR | 2μl
|
| dNTP | 2μl
|
| Buffer | 5μl
|
| ddH2O | 39.25μl
|
| Enzyme tag | 0.25μl
|
|
- Liquid culture 12:00
- the first step when we added MX1 we didn't resuspend,so we readded MX1~MX3.But the DNA may be less.
- digest
- [GFP(XP),ribo1(SP,XP>>use 10 ul and 20ul protocal),ribo2(SP,XP>>use 10 ul and use 20ul protocal)]
| Total: | 10μl
|
| DNA of vector(sp) | 1μl
|
| DNA of insert(xp) | 3μl
|
| buffer | 2μl
|
| ligase | 0.5μl
|
| ddH2O | 4.5μl
|
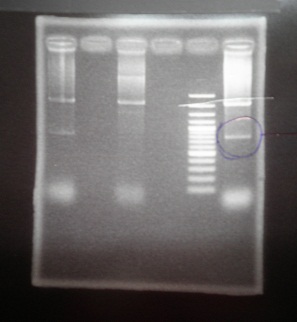 |
| Descr | Win length | Fail length
|
| 1: ribo1 | 96bp |
|
| 2: | |
|
| 3: ribo2 | 96bp |
|
| 4: | |
|
| 5: Marker:100bp | |
|
| 6: GFP | 854bp |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| Total:(*2) | 50μl
|
| template | 2μl
|
| VF+VR | 2μl
|
| dNTP | 2μl
|
| Buffer | 5μl
|
| ddH2O | 39.75μl
|
| Enzyme tag | 0.25μl
|
|
- purify(ribo-1(sp);ribo-2(sp);GFP(xp))
- ligate
- transform
- liquid culture from the plate(8/24)
2010.08.27
- PCR mix 10:30
- GFP+terminator+Riboswitch=1157bp
- 3 in 1 12:15
- Run PCR 12:30~13:30
- Run gel 13:45
- Liquid culture again(use the plate 8/24) 10:30
- To check the wrong step of liquid culture
- Digestion 13:40
- Run gel 01:45pm[GFP(XP).Riboswitch-1(XP).Riboswitch-2(XP)]
| File:NYMU R27.jpg |
"
| Descr | Win length | Fail length
|
| 1: | - | -
|
| 2: riboswitch+GFP | 1167bp | 292bp
|
| 3: riboswitch+GFP | 1167bp | 292bp
|
| 4: | - | -
|
| 5: Positive Control | 1100 bp | none
|
| 6: | - | -
|
| 7: Negative Control | | Contamination ~1000bp
|
| 8: | - | -
|
| | | n
|
| 10: | - | -
|
| 11: GFP(XP) | 879bp | 854bp
|
| 12: | - | -
|
| 13: ribo-1(xp) | 292bp |
|
| 14: | - | -
|
| 15: ribp-2(xp) | 292bp |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| Total:(*2) | 50μl
|
| template | 2μl
|
| VF+VR | 2μl
|
| dNTP | 2μl
|
| Buffer | 5μl
|
| ddH2O | 39.75μl
|
| Enzyme tag | 0.25μl
|
|
2010.08.28
- 3-IN-1 (another 3 colony from 08.26 Riboswitch+GFP-Terminator plate) 13:30
- PCR (Use new dNTP & Taq buffer & taq) 14:30
- Run Gel 16:20
 |
| Descr | Win length | Fail length
|
| 1: riboswitch+GFP | 1167bp | 292bp
|
| 2: riboswitch+GFP | 1167bp | 292bp
|
| 3: riboswitch+GFP | 1167bp | 292bp
|
| 4: positive control | 1100bp | -
|
| 5: marker100 bp | - | -
|
| 6: negative control | - | -
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| Total:(*2) | 50μl | temp | time
|
| template | 2μl | 94oC | 60s
|
| VF+VR | 2μl | 94oC | 15s
|
| dNTP | 2μl | 55oC | 20s
|
| Buffer | 5μl | 72oC | 100s
|
| ddH2O | 39.75μl | 72oC | 300s
|
| Enzyme tag | 0.25μl
|
|
- Transformation GFP+vector 17:00
- 3-IN-1 (2 Colony from the plate transformed on 08/27) 17:20
2010.08.29
- Run Gel (with green marker) at 11:00
- Ribo Vac1 and Ribo Vac2 from PCR on 8/28 at 18:25~19:40
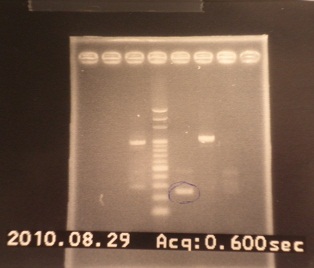 |
| Descr | Win length | Fail length
|
| 1: - | - | -
|
| 2: - | - | -
|
| 3: riboswitch1+vector | 292bp | -
|
| 4: marker100 bp | - | -
|
| 5: riboswitch2+vector | 292bp | -
|
| 6: positive control | 1100bp | -
|
| 7: negative control | - | -
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| Total:(*2) | 50μl
|
| template | 2μl
|
| VF+VR |
|
| dNTP | 2μl
|
| Buffer | 5μl
|
| ddH2O | 39.75μl
|
| Enzyme tag | 0.25μl
|
|
______________________________________________________________________________________________________________
- 3-in-1 (3 colonies of GFP+TERMINATOR and 1 of Riboswitch+PSB1A2 vector, both from plates transformed on 8/28)
- PCR 11:00~12:50
- Run Gel 15:30 (result shows no band!! but positive and negative controls are correct)
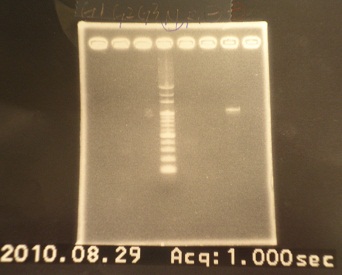 |
| Descr | Win length | Fail length
|
| 1: GFP1 | 854bp |
|
| 2: GFP2 | 854bp |
|
| 3: GFP3 | 854bp |
|
| 4: marker100 bp | - | -
|
| 5: riboswitch+vector | 292bp | -
|
| 6: negative control | - | -
|
| 7: positive contril | 1100bp | -
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| Total:(*2) | 50μl
|
| template | 2μl
|
| VF+VR | 2μl
|
| dNTP | 2μl
|
| Buffer | 5μl
|
| ddH2O | 39.75μl
|
| Enzyme tag | 0.25μl
|
|
- transform 16:30
- (1)the Ribo2 from 8.17 (2)the Ribo (check) from 08/19 (3)GFP+terminator from 08/27
2010.08.30
- 3-in-1: GFP,GFP+riboswitch,ribo-1,ribo-2 12:30pm
- Run PCR 13:20
- Run gel(2% argarose 100v)
|
|
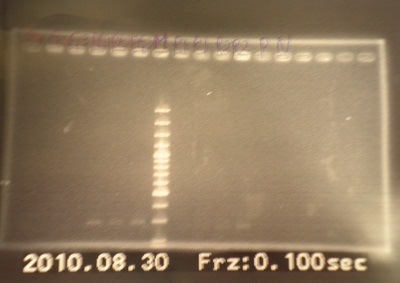 |
| Descr | Win length | Fail length
|
| 1: GFP1 | 854bp | -
|
| 2: GFP2 | 854bp | -
|
| 3: GFP3 | 854bp | -
|
| 4: riboswitch1+GFP | 1167bp | -
|
| 5: riboswitch2+GFP | 1167bp | -
|
| 6: riboswitch3+GFP | 1167bp | -
|
| 7: marker100 bp | - | -
|
| 8: ribo1+vector | 292bp | -
|
| 9: ribo1+vector | 292bp | -
|
| 10: ribo2+vector | 292bp | -
|
| 11: ribo2+vector | 292bp | -
|
| 12: positive control | 1100bp | -
|
| 13: negative control | - | -
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| Total:(*2) | 50μl
|
| template | 2μl
|
| VF+VR |
|
| dNTP | 2μl
|
| Buffer | 5μl
|
| ddH2O | 39.75μl
|
| Enzyme tag | 0.25μl
|
|
- Finally we Transform 19F(GFP+terminator), E0040 18:50
2010.08.31
- 3-in-1 (ribo(xp)+pSB1A2,E0040,J04630)11:00
- Nanodrop
- Ligation
|
|
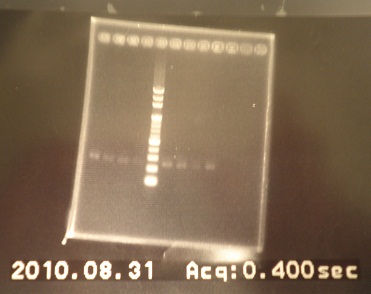 |
| Descr | Win length | Fail length
|
| 1: GFP1(J04630) | 854bp | -
|
| 2: GFP2(J04630) | 854bp | -
|
| 3: Riboswitch1+vector | 292bp | -
|
| 4: Riboswitch+2vector | 292bp | -
|
| 5: marker100 bp | - | -
|
| 6: GFP1(E0040) | 720bp | -
|
| 7: GFP2(E0040) | 720bp | -
|
| 8: ribo1+vector | 292bp | -
|
| 9: ribo1+vector | 292bp | -
|
| 10: ribo2+vector | 292bp | -
|
| 11: ribo2+vector | 292bp | -
|
| 12: positive control | 1100bp | -
|
| 13: negative control | - | -
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| Total:(*2) | 50μl
|
| template | 2μl
|
| VF+VR |
|
| dNTP | 2μl
|
| Buffer | 5μl
|
| ddH2O | 39.75μl
|
| Enzyme tag | 0.25μl
|
|
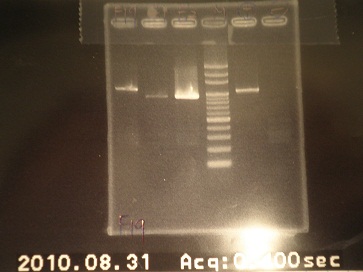 |
| Descr | Win length | Fail length
|
| 1: GFP+terminator(19f) | 854bp | -
|
| 2: GFP(E0040) | 720bp | -
|
| 3: GFP(E0040) | 720bp | -
|
| 4: marker 100bp | - | -
|
| 5: Positive Control | 1100 bp |
|
| 6: Negative Control | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
|
2010.09.01
- GFP*3 Plasmid Extraction 10:30
- Digestion 12:30 (13:20)
- Run gel 15:30
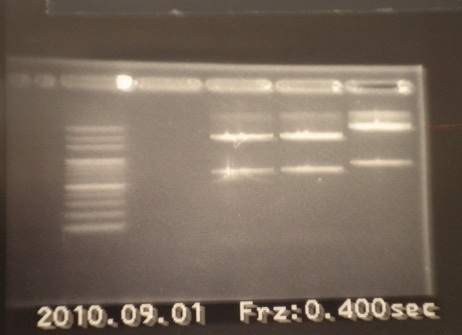 |
| Descr | Win length | Fail length
|
| 1: - | - | -
|
| 2: - | - | -
|
| 3: marker 100bp | - | -
|
| 4: - | - | -
|
| 5: (GFP)E0040-2 | 745bp |
|
| 6: (GFP)E0040-1 | 745bp |
|
| 7: (GFP)J04630 | 856bp |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| Total:(*2) | 50μl
|
| template | 2μl
|
| VF+VR | 2μl
|
| dNTP | 2μl
|
| Buffer | 5μl
|
| ddH2O | 39.25μl
|
| Enzyme tag | 0.25μl
|
|
- RG1,RG2,RG3,PR1,PR2,PR3 3-in-1 10:00
- Colony PCR 11:00~13:00
- Run Gel
 |
"
| Descr | Win length | Fail length
|
| 1: riboswitch+GFP(1-a) | 1167bp | 292bp
|
| 2: riboswitch+GFP(1-b) | 1167bp | 292bp
|
| 3: riboswitch+GFP(2-a) | 1167bp | 292bp
|
| 4: riboswitch+GFP(2-b) | 1167bp | 292bp
|
| 5: riboswitch+GFP(3-a) | 1167bp | 292bp
|
| 6: riboswitch+GFP(3-b) | 1167bp | 292bp
|
| 7: - | - | -
|
| 8: marker 100bp | - | -
|
| 9: promoter+ribo+GFP | bp | -
|
| 10: promoter+ribo+GFP | bp | -
|
| 11: promoter+ribo+GFP | bp | -
|
| 12: promoter+ribo+GFP | bp | -
|
| 13: promoter+ribo+GFP | bp | -
|
| 14: promoter+ribo+GFP | bp | -
|
| 15: positive control | 1100bp | -
|
| 16: negative control | - | containment
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| Total:(*2) | 50μl | temp | time
|
| template | 2μl | 94 | 60s
|
| VF+VR | 2μl | 94 | 15s
|
| dNTP | 2μl | 55 | 20s
|
| Buffer | 5μl | 72 | 90s
|
| ddH2O | 39.75μl | 72 | 300s
|
| Enzyme tag | 0.25μl
|
|
2010.09.02
- Liquid culture plasmid extraction 10:00
- Nanodrop
- Digestion 12:12
- Purification
- Nanodrop
- Ligation
- Transform 17:00
2010.09.03
- 3-in-1 chose 4 colony
- PCR 11.20
- run gel
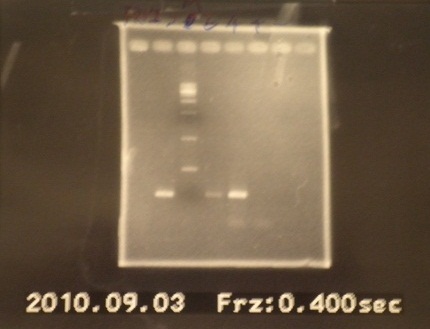 |
| Descr | Win length | Fail length
|
| 1: FRV1 | 1167bp | -
|
| 2: FRV2 | 1167bp | -
|
| 3: marker 100bp | - | -
|
| 4: FRV3 | - | -
|
| 5: FRV4 | 745bp |
|
| 6: positive control | 1100bp |
|
| 7: negative control | | contamination~3000bp
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| Total:(*2) | 50μl
|
| template | 2μl
|
| VF+VR | 2μl
|
| dNTP | 2μl
|
| Buffer | 5μl
|
| ddH2O | 39.75μl
|
| Enzyme tag | 0.25μl
|
|
(the marker takes the wrong one,1kb marker is correct)
-
- ligate again(insert+vector=8.5)
- transform 16:40
2010.09.04
- PCR 20:35pm (3 colony from 20100903 plate)
- run gel(FRV1&2&3&+&-) 100V 35min
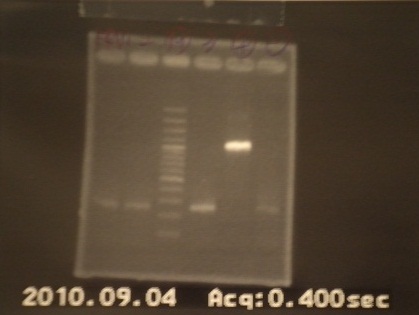 |
| Descr | Win length | Fail length
|
| 1: FRV1 | 1167bp | -
|
| 2: FRV2 | 1167bp | -
|
| 3: marker 100bp | - | -
|
| 4: FRV3 | - | -
|
| 5: FRV4 | 745bp |
|
| 6: positive control | 1100bp |
|
| 7: negative control | | contamination~3000bp
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| Total:(*2) | 50μl
|
| template | 2μl
|
| VF+VR | 2μl
|
| dNTP | 2μl
|
| Buffer | 5μl
|
| ddH2O | 39.75μl
|
| Enzyme tag | 0.25μl
|
|
2010.09.05
- digest again(because we used XP's protocol for SP)
- purification at 14:25
- nanodrop at 15:00
- we digest another tube (ribo2-2 SP) 15:50
- ligation
- transform 17:00
2010.09.06
- Colony PCR 11:30
- Run gel 14:30(2% argarose 100v 25 mins)
|
|
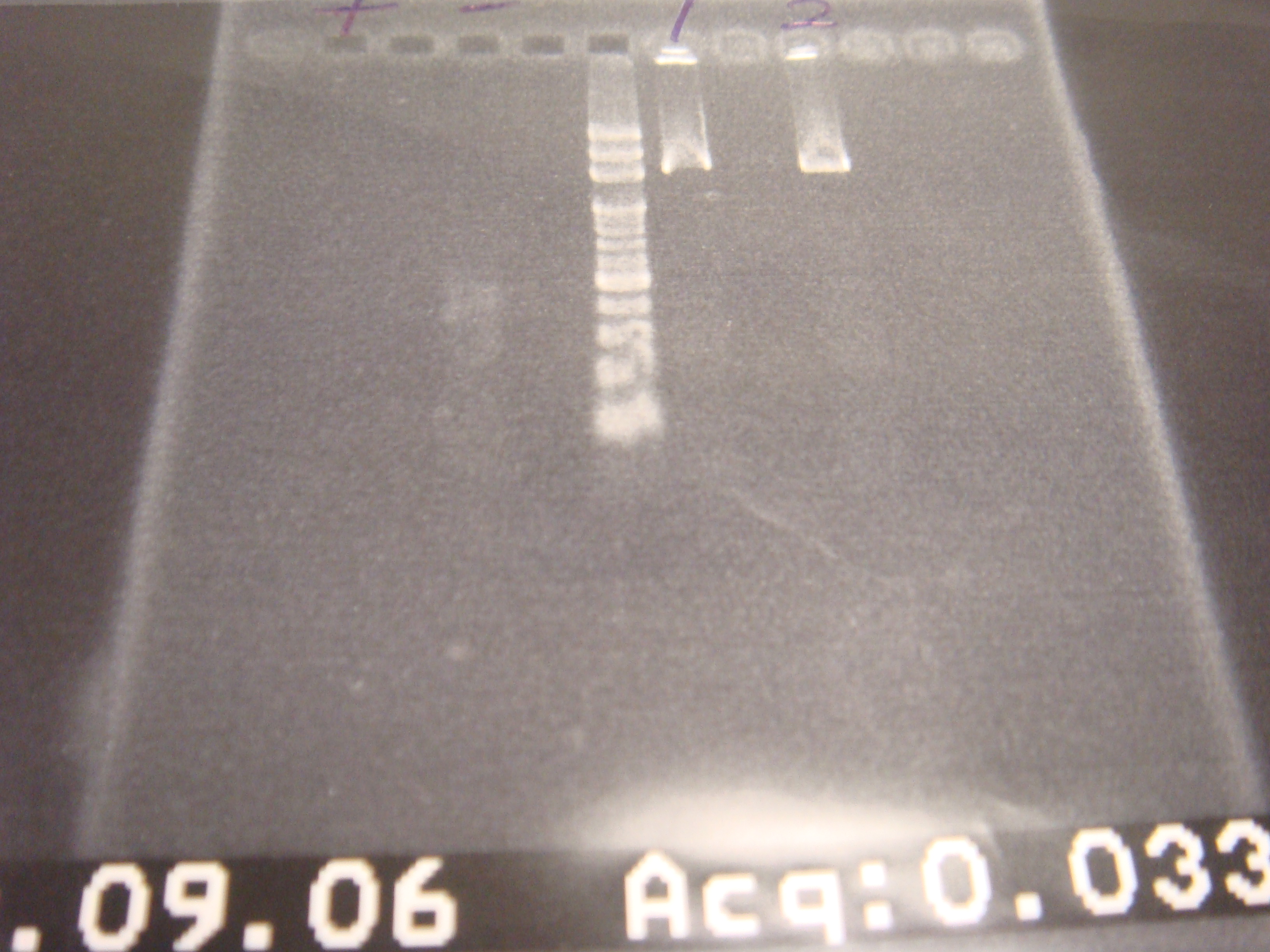 |
"
| Descr | Win length | Fail length
|
| 1: riboswitch+GFP | 1167bp | 292bp
|
| 2: riboswitch+GFP | 1167bp | 292bp
|
| 3: riboswitch+GFP | 1167bp | 292bp
|
| 4: riboswitch+GFP | 1167bp | 292bp
|
| 5: riboswitch+GFP | 1167bp | 292bp
|
| 6: riboswitch+GFP | 1167bp | 292bp
|
| 7: marker 100bp | - | -
|
| 8: promoter+ribo+GFP | bp | -
|
| 9: ribo1+vector | 292bp | -
|
| 10: ribo1+vector | 292bp | -
|
| 11: ribo2+vector | 292bp | -
|
| 12: ribo2+vector | 292bp | -
|
| 13: ribo3+vector | 292bp | -
|
| 14: positive control | 1100bp | -
|
| 15: negative control | - | containment
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| Total:(*2) | 50μl
|
| template | 2μl
|
| VF+VR |
|
| dNTP | 2μl
|
| Buffer | 5μl
|
| ddH2O | 39.75μl
|
| Enzyme tag | 0.25μl
|
|
- Take out the digest(sp)yesterday 10:30
- digest purify
- nanodrop(13.2ng/nl)
- ligate 11:40~14:30
- transform 17:00
2010.09.07
- 3-in-1 10:30am
- Run PCR 10:45am
|
|
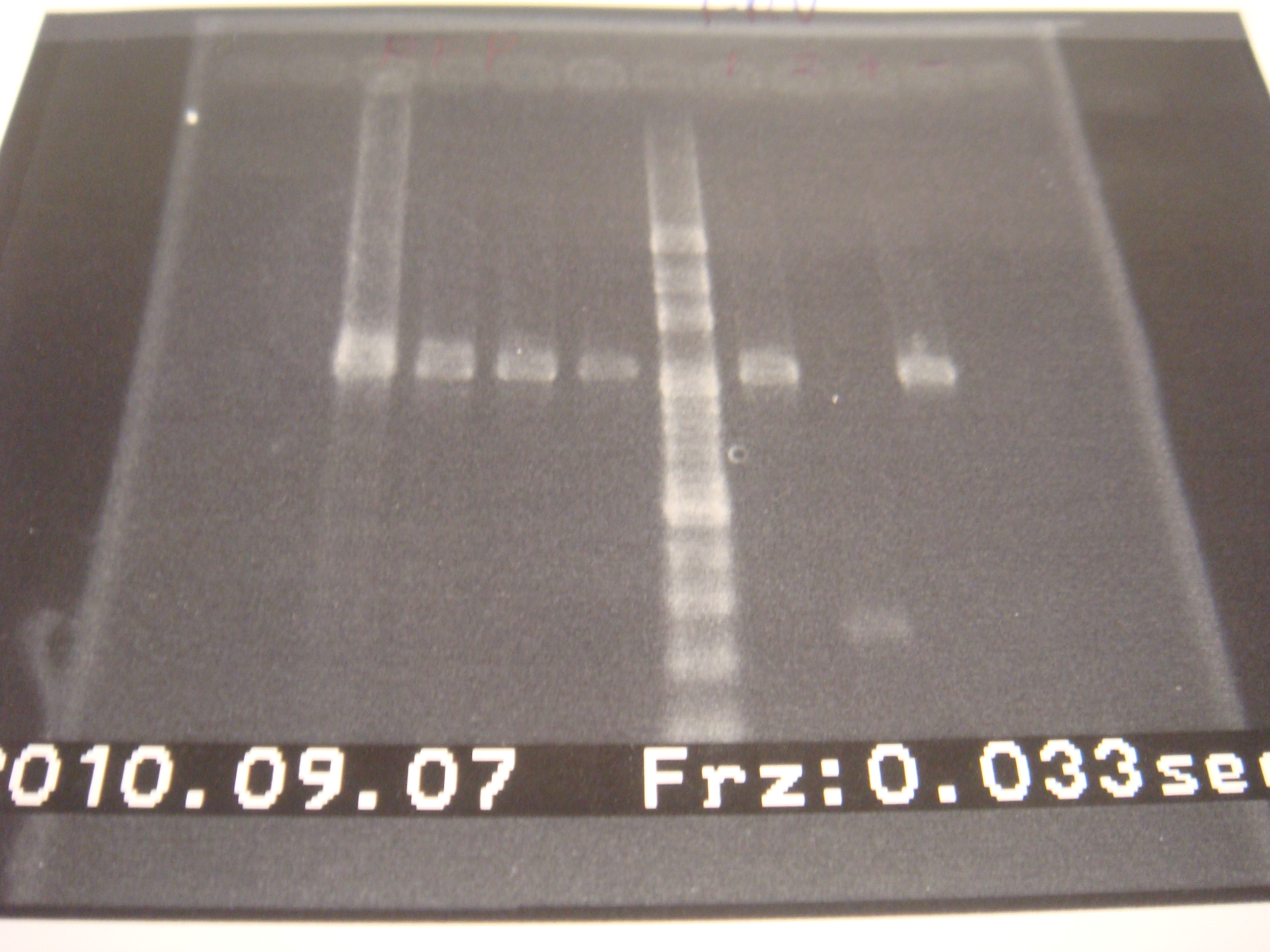 |
"
| Descr | Win length | Fail length
|
| 1: riboswitch+GFP | 1167bp | 292bp
|
| 2: riboswitch+GFP | 1167bp | 292bp
|
| 3: riboswitch+GFP | 1167bp | 292bp
|
| 4: riboswitch+GFP | 1167bp | 292bp
|
| 5: riboswitch+GFP | 1167bp | 292bp
|
| 6: riboswitch+GFP | 1167bp | 292bp
|
| 7: marker 100bp | - | -
|
| 8: promoter+ribo+GFP | bp | -
|
| 9: ribo1+vector | 292bp | -
|
| 10: ribo1+vector | 292bp | -
|
| 11: ribo2+vector | 292bp | -
|
| 12: ribo2+vector | 292bp | -
|
| 13: ribo3+vector | 292bp | -
|
| 14: positive control | 1100bp | -
|
| 15: negative control | - | containment
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| Total:(*2) | 50μl
|
| template | 2μl
|
| VF+VR |
|
| dNTP | 2μl
|
| Buffer | 5μl
|
| ddH2O | 39.75μl
|
| Enzyme tag | 0.25μl
|
|
2010.09.08
- plasmid extraction
- digestion(FRV1(XP)) 11:10
- ligation 14:00
- Transformation 17:00
2010.09.09
- Because we didn't cut gel(Ribo+GFP:XP), we restart again.
- Re-Digest
| File:NYMU P9070327.JPG |
| Descr | Win length | Fail length
|
| 1: Real PCR :Riboswitch | 56 bp | -
|
| 2: marker 100bp | - | -
|
| 3: positive control | 200bp |
|
| 4: negative control | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| | |
|
| Total:(*2) | 50μl
|
| template | 2μl
|
| VF+VR | 2μl
|
| dNTP | 2μl
|
| Buffer | 5μl
|
| ddH2O | 39.75μl
|
| Enzyme tag | 0.25μl
|
|
2010.09.10
- real PCR
- run gel
- cut gel
- gel extraction
- digest(xp)
- nanodrop
- ligate
- transform
2010.09.11
- two real tubes run gel and cut gel and gel extraction
2010.09.12
- run gel(length isn't correct)
- (real PCR) digestion 12:00
- ligation 15:00
- transform 19:40
2010.09.13
- colony PCR 13:25 (3 in 1)
- nanodrop
- ligation 14:00
- Colony PCR 15:00
2010.09.22
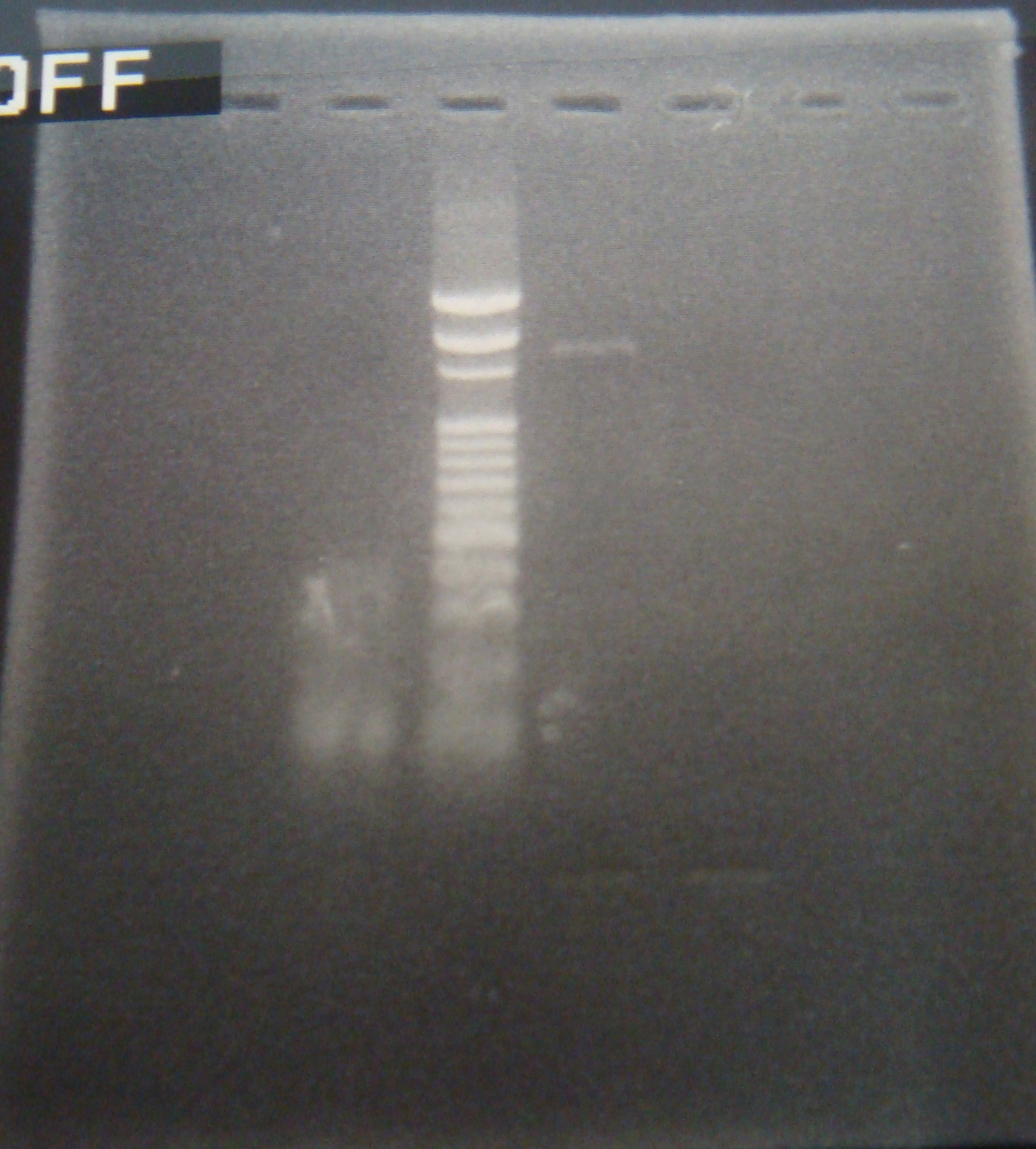 |
| Total: | 49μl | X1.7μl |
|
| FP | 1μl | 1.7μl | Theophylline Riboswitch Forward Primer
|
| RP | 1μl | 1.7μl | Theophylline Riboswitch Reverse Primer
|
| dNTP | 2μl | 3.4μl |
|
| 10XBuff. | 5μl | 8.5μl |
|
| pfu | 0.25μl | 0.425μl |
|
| ddH2O | 39.75μl | 67.575μl |
|
| PCR Protocol |
|
| 94
| 60s
|
| 94
| 15s
|
| 52
| 20s
|
| 68
| 30s
| 35 cycles
|
| 68
| 300s
|
|
- Digest ribo(xp) overnight
- Ligate ribo with vector(xp)
- Transform:23:30
2010.09.23
- Ligate ribo with vector again
- Transform
2010.09.24
- 3-in-1 (ribo+vector)
- colony PCR
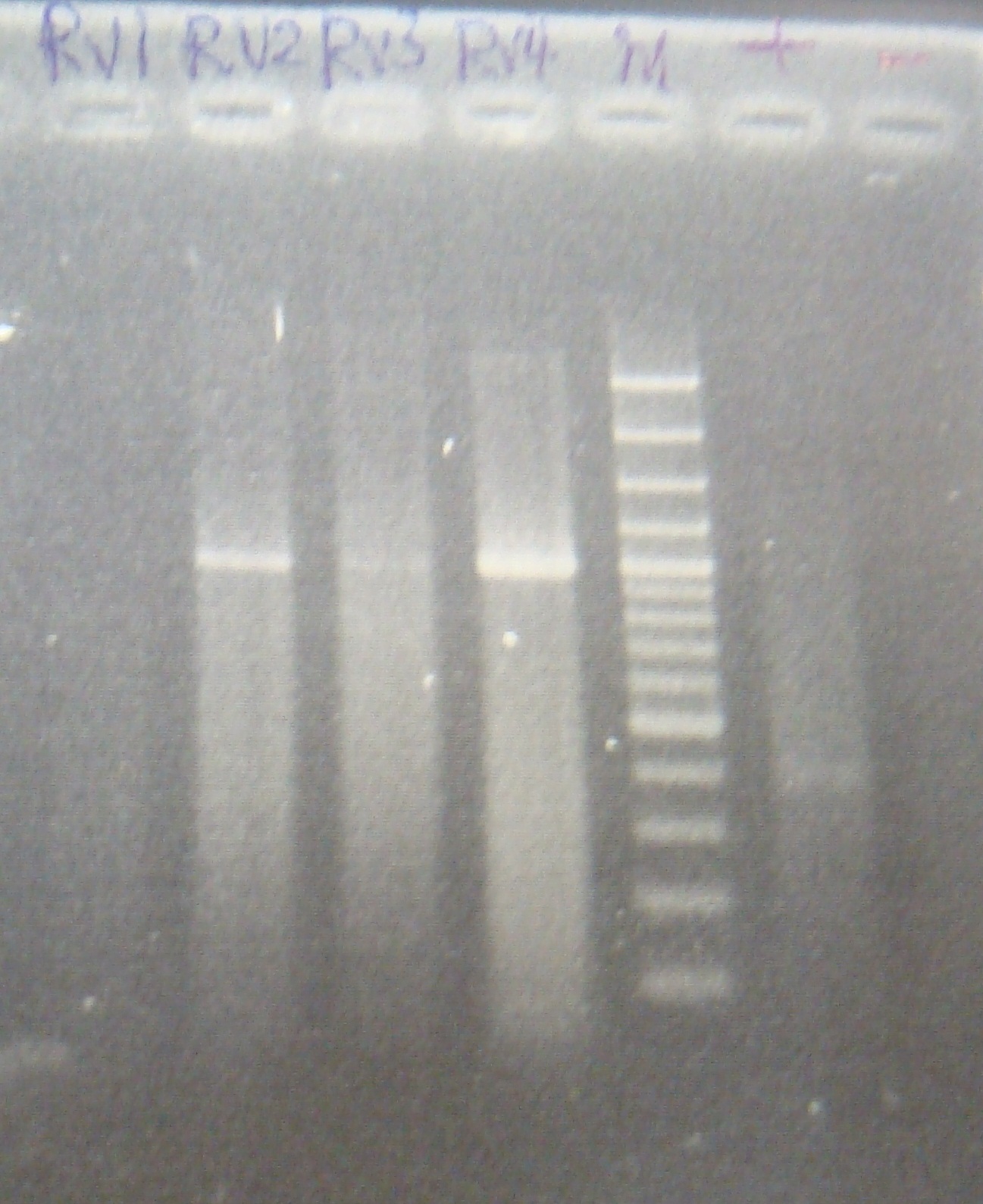 |
| Total: | 49μl | X1.9μl
|
| VR+VF | 2μl | 3.8μl
|
| dNTP | 2μl | 3.8μl
|
| 10XBuff. | 5μl | 9.5μl
|
| tag | 0.25μl | 0.475μl
|
| ddH2O | 39.75μl | 75.525μl
|
| PCR Protocol |
|
| 94
| 60s
|
| 94
| 15s
|
| 55
| 20s
|
| 72
| 30s
| 30 cycles
|
| 72
| 300s
|
|
- Cut gel(ribo&pSB1A2(xp) digested)


2010.09.26
- 3-in-1(0925 plate)
- colony PCR
 |
| Total: | 49μl | X2.5μl
|
| VR+VF | 2μl | 5μl
|
| dNTP | 2μl | 5μl
|
| 10XBuff. | 5μl | 12.5μl
|
| tag | 0.25μl | 1.25mu;l
|
| ddH2O | 39.75μl | 99.375μl
|
| PCR Protocol |
|
| 94
| 60s
|
| 94
| 15s
|
| 55
| 20s
|
| 72
| 30s
| 30 cycles
|
| 72
| 300s
|
|
2010.09.27
- plasmid extraction(RV)
- Digest RV 13:30
- run gel
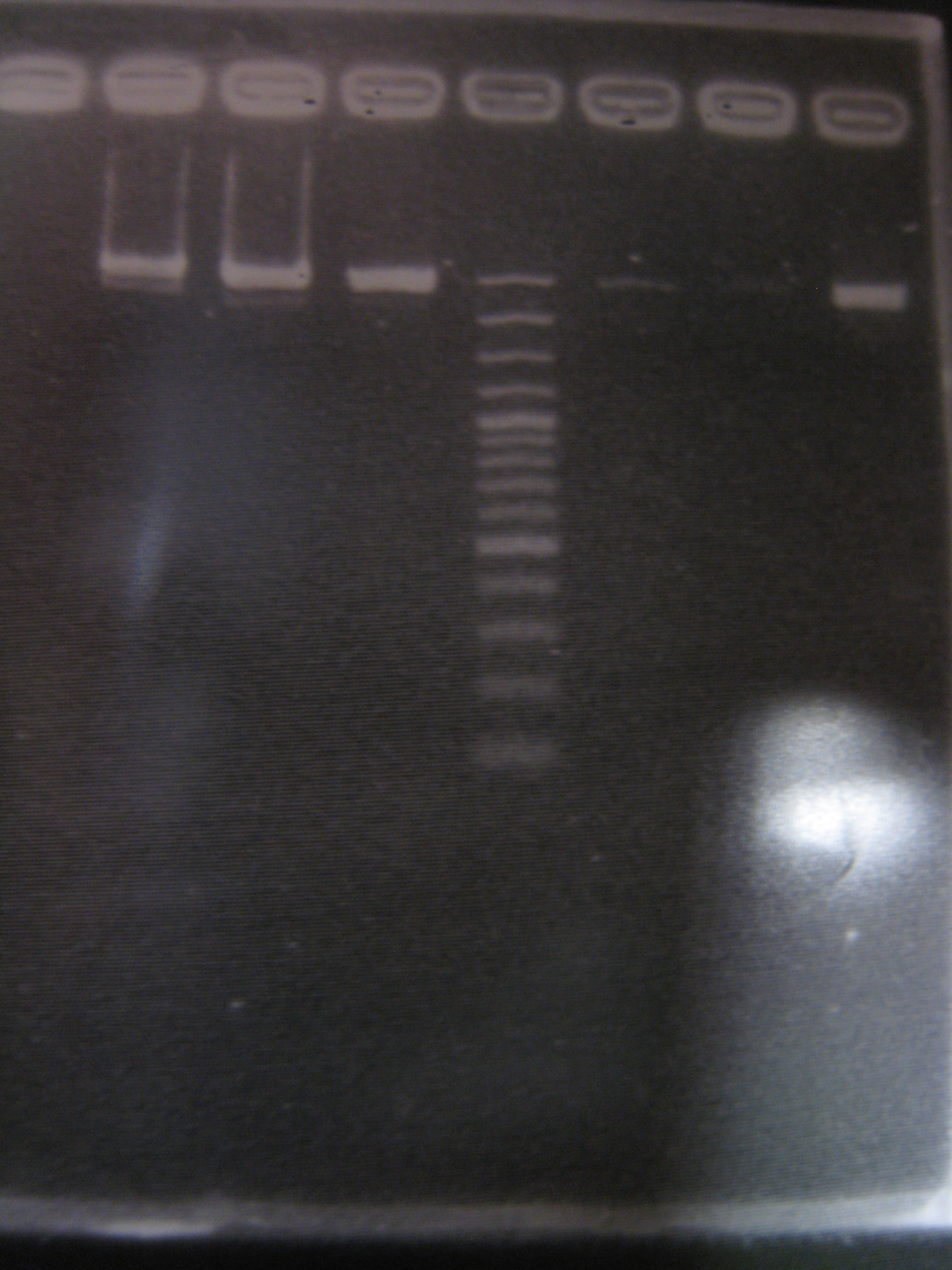
There is GFP on vector, so it's wrong.
- Digest ribo again overnight
2010.09.28
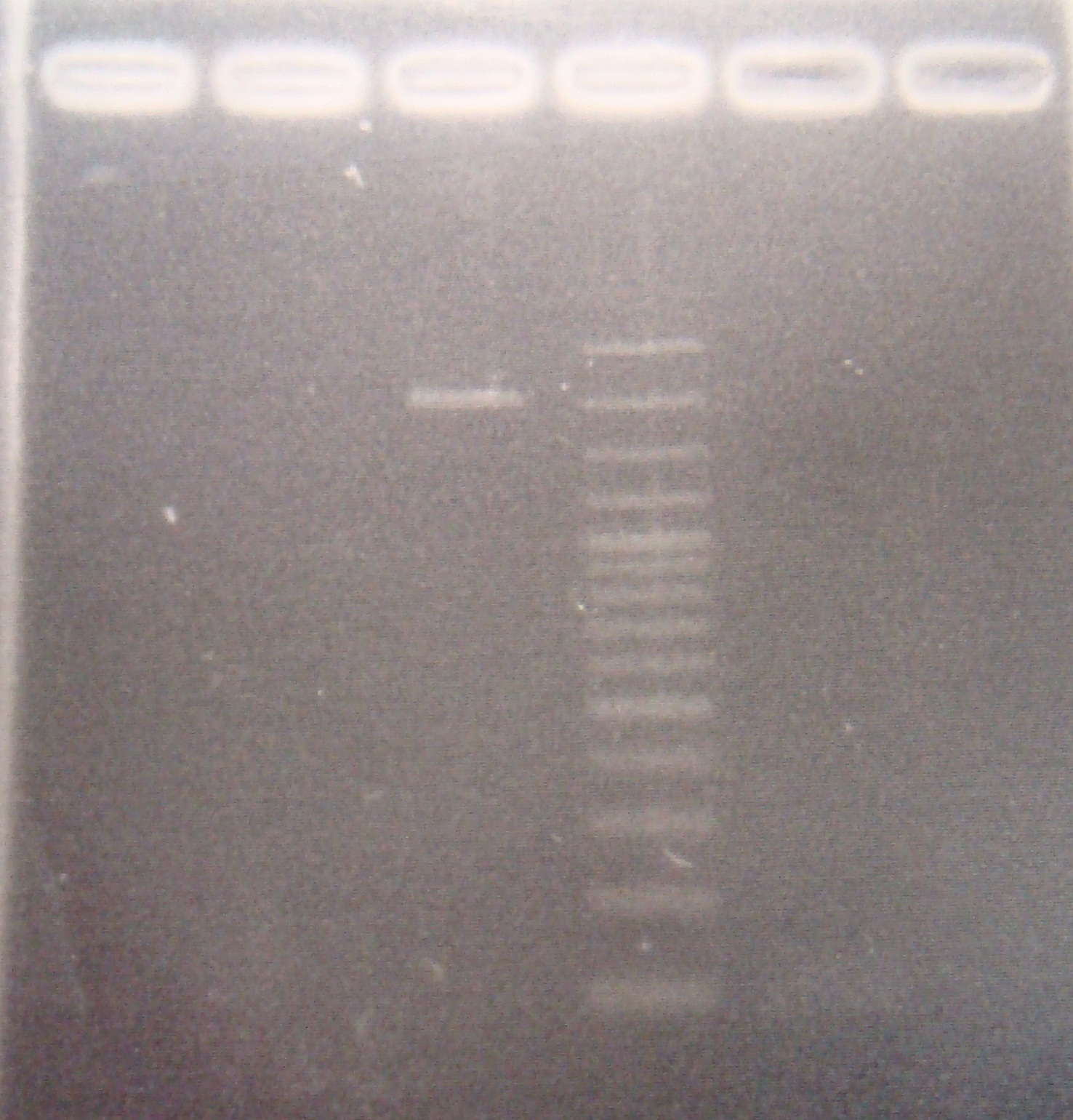
- Ligate ribo with vector
- Transform
According to 0927 gel pic, 30th colony may be correct RV, so we take out second plate and incubate in LB liguid.
- plasmid extraction 21:30
- Digest RV(SP) overnight
2010.09.29
- Ligate RV(sp) with GFP(BBa_J04630 already have terminator)
- Transform
2010.09.30
2010.10.01
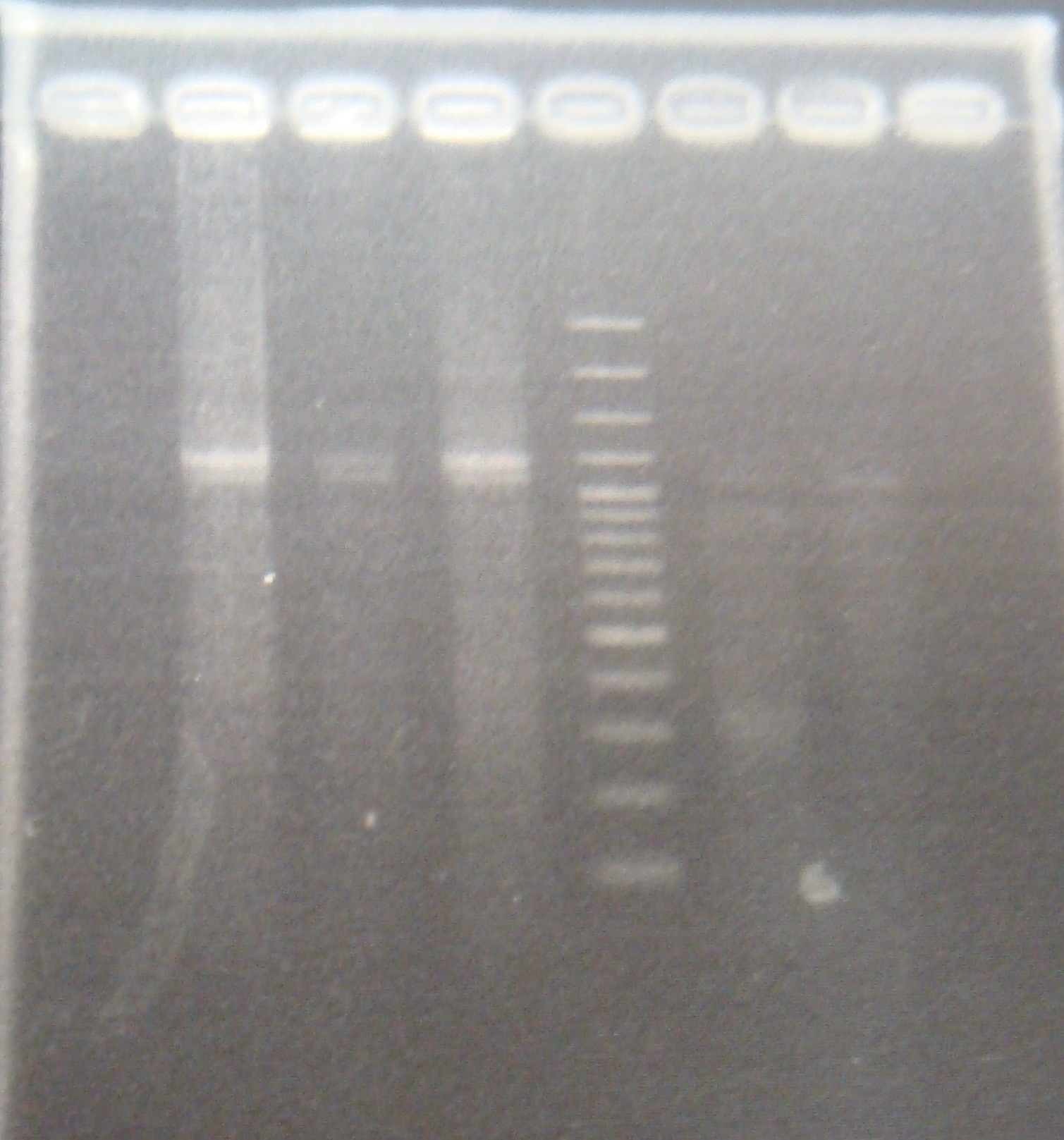 |
| Total: | 49μl | X1.6μl
|
| VR+VF | 2μl | 3.2μl
|
| dNTP | 2μl | 3.2μl
|
| 10XBuff. | 5μl | 8μl
|
| tag | 0.25μl | 0.4mu;l
|
| ddH2O | 39.75μl | 63.6μl
|
| PCR Protocol |
|
| 94
| 60s
|
| 94
| 15s
|
| 55
| 20s
|
| 72
| 70s
| 30 cycles
|
| 72
| 300s
|
|
2010.10.02
- BBa_J04630 plasmid extraction
- Digest J04630(XP)
- cut gel(GFP)
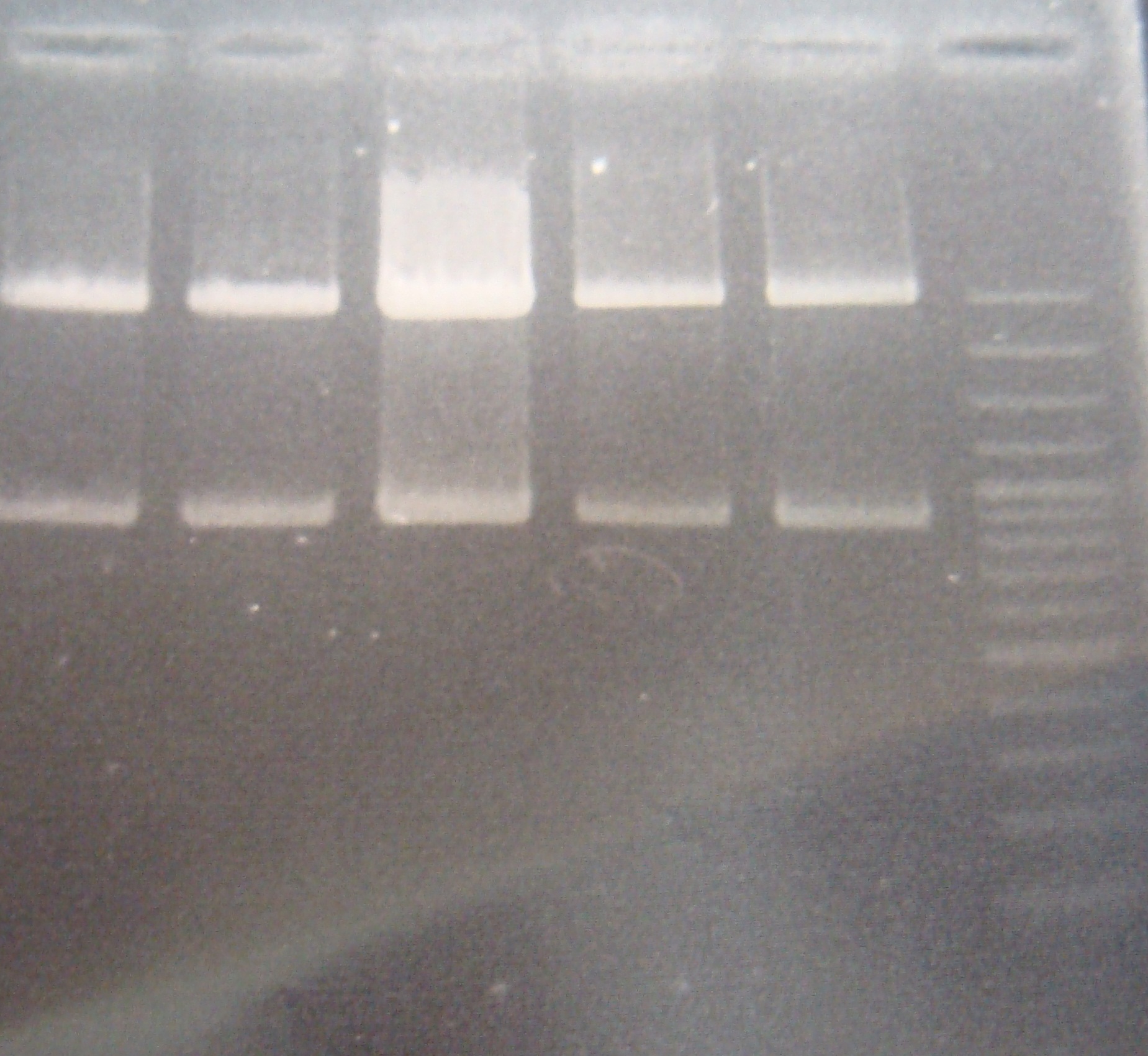
- Ligate RV(SP) with GFP(XP)
- Transform
2010.10.03
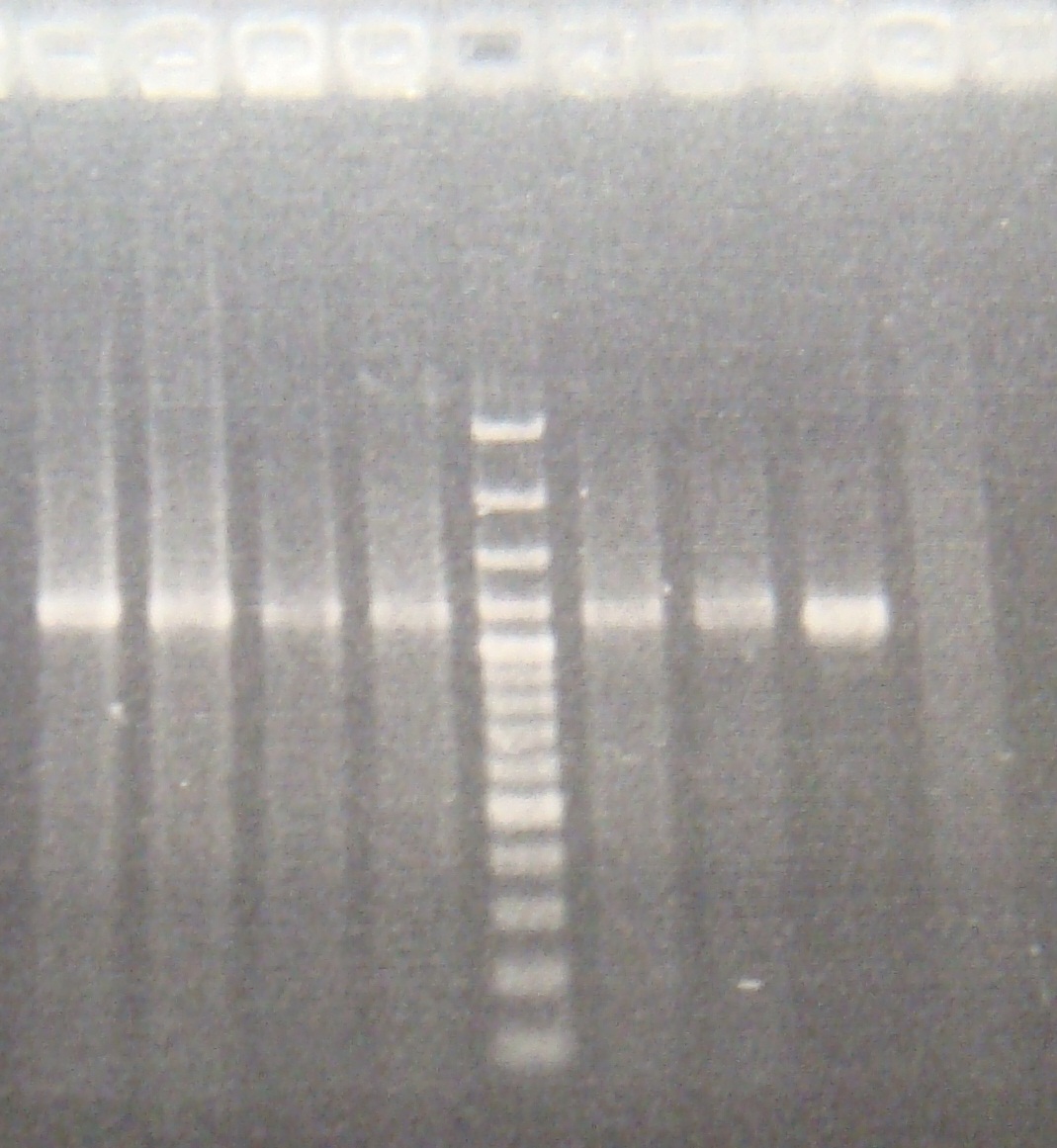 |
| Total: | 49μl | X1,9μl
|
| VR+VF | 2μl | 3.8μl
|
| dNTP | 2μl | 3.8μl
|
| 10XBuff. | 5μl | 9.5μl
|
| tag | 0.25μl | 0.475mu;l
|
| ddH2O | 39.75μl | 75.525μl
|
| PCR Protocol |
|
| 94
| 60s
|
| 94
| 15s
|
| 55
| 20s
|
| 72
| 100s
| 30 cycles
|
| 72
| 300s
|
|
2010.10.04
- FRV plasmid extraction
- Digest FRV (XP)
- cut gel & purify(FRV)
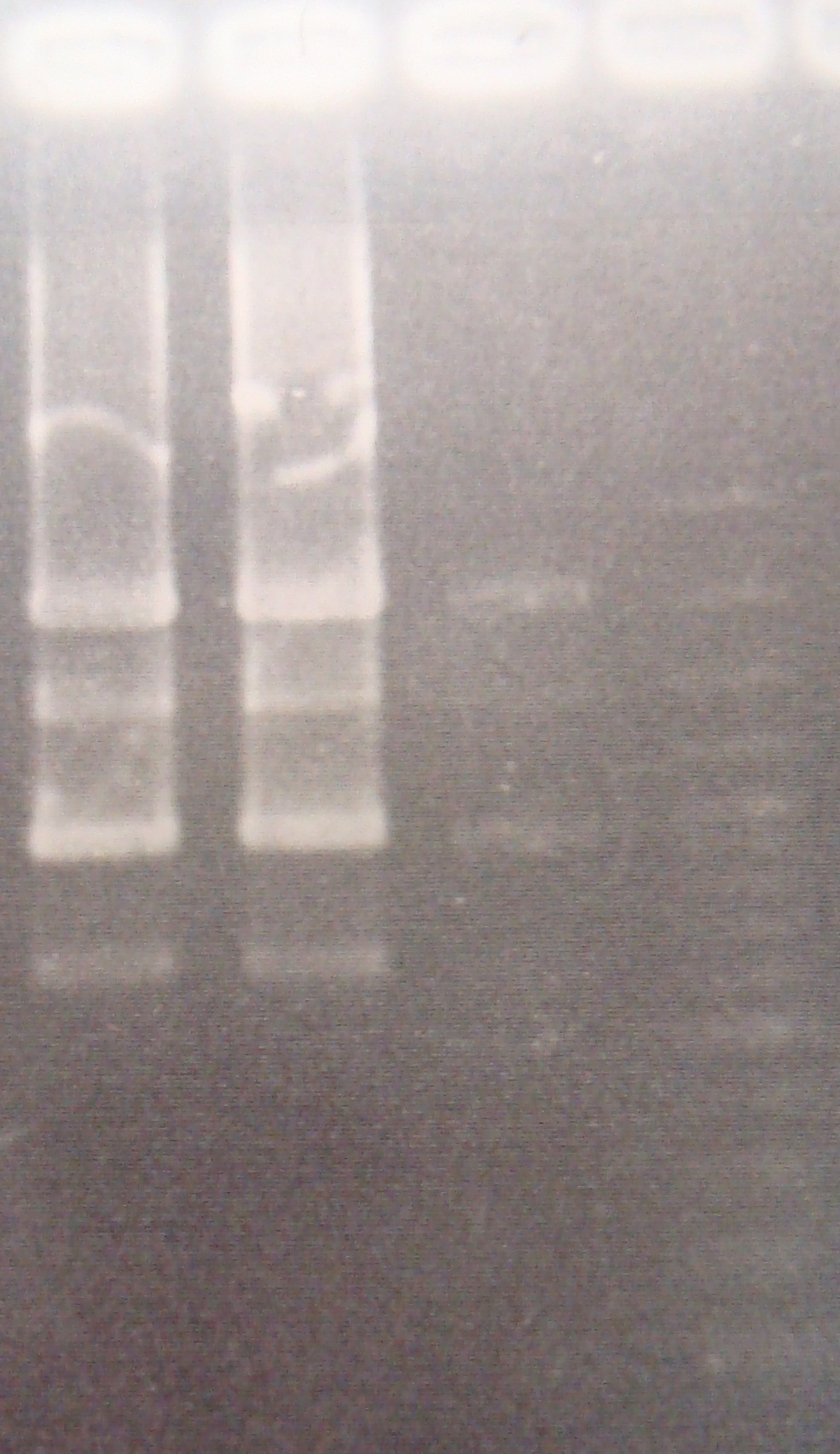
- Ligate FRV with pLac(from ssrA group)
- Transform
- pLac plasmid extraction
- Digest pLac(SP) overnight
2010.10.05
- pLac digest purify(run gel with 3-in-1)
- PFRV, GFP, RV 3-in-1
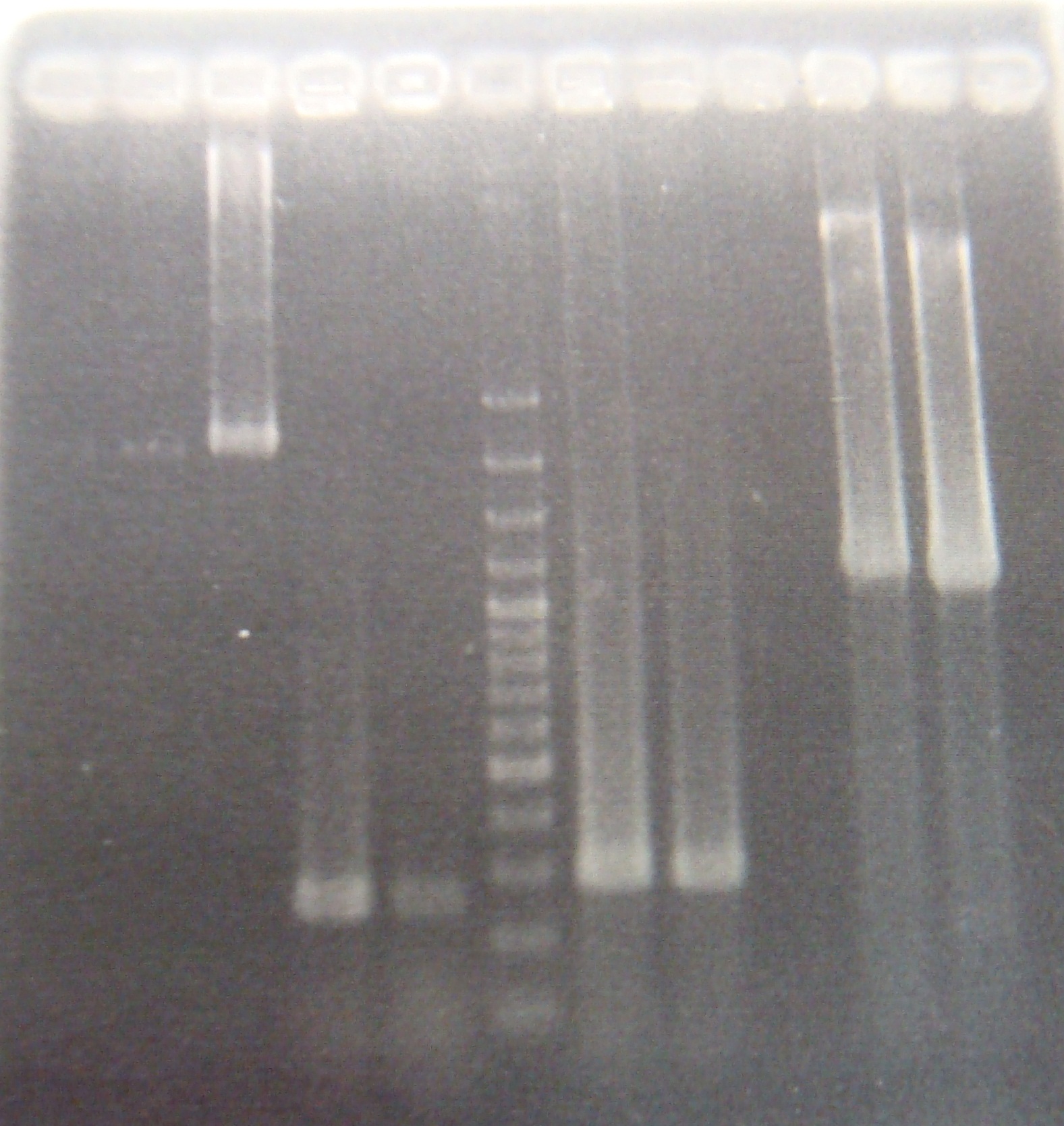 |
Total: !! 49μl || X2.5μl
| VR+VF | 2μl | 5μl
|
| dNTP | 2μl | 5μl
|
| 10XBuff. | 5μl | 12.5μl
|
| tag | 0.25μl | 0.625mu;l
|
| ddH2O | 39.75μl | 99.375μl
|
| PCR Protocol |
|
| 94
| 60s
|
| 94
| 15s
|
| 55
| 20s
|
| 72
| 90s
| 30 cycles
|
| 72
| 300s
|
|
- Digest pLac(SP) again overnight
2010.10.06
- Ligate pLac(SP) with FRV(XP)
- Transform 22:40
2010.10.07
- Ligate FRV with pLac
- Transform
2010.10.08
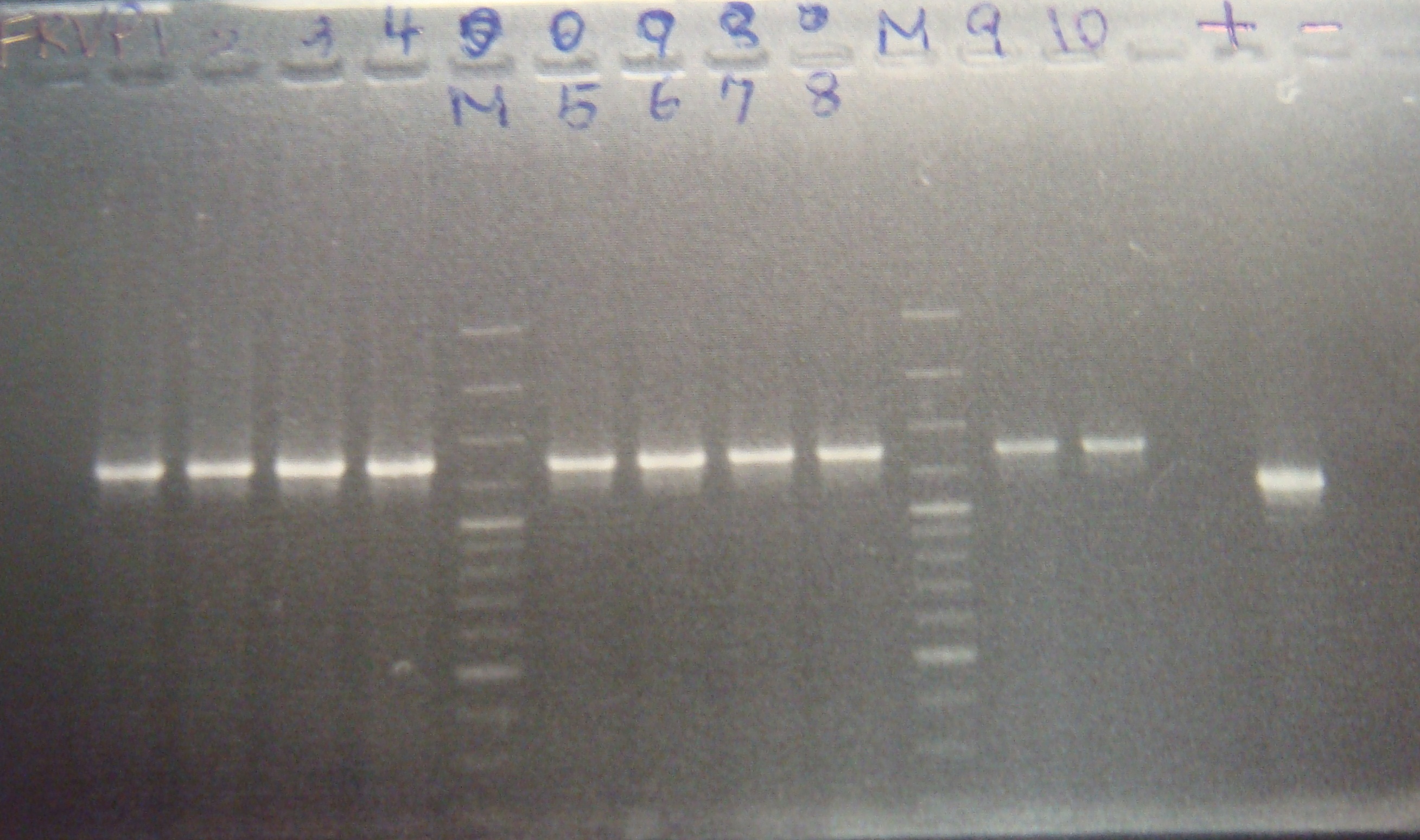 |
| Total: | 49μl | 3.8μl
|
| VR+VF | 2μl | μl
|
| dNTP | 2μl | 3.8μl
|
| 10XBuff. | 5μl | 9.5μl
|
| tag | 0.25μl | 0.475mu;l
|
| ddH2O | 39.75μl | 75.525μl
|
| PCR Protocol |
|
| 94
| 60s
|
| 94
| 15s
|
| 55
| 20s
|
| 72
| 100s
| 30 cycles
|
| 72
| 300s
|
|
- Ligate FRV with pLac
- Transform 22:15
2010.10.09
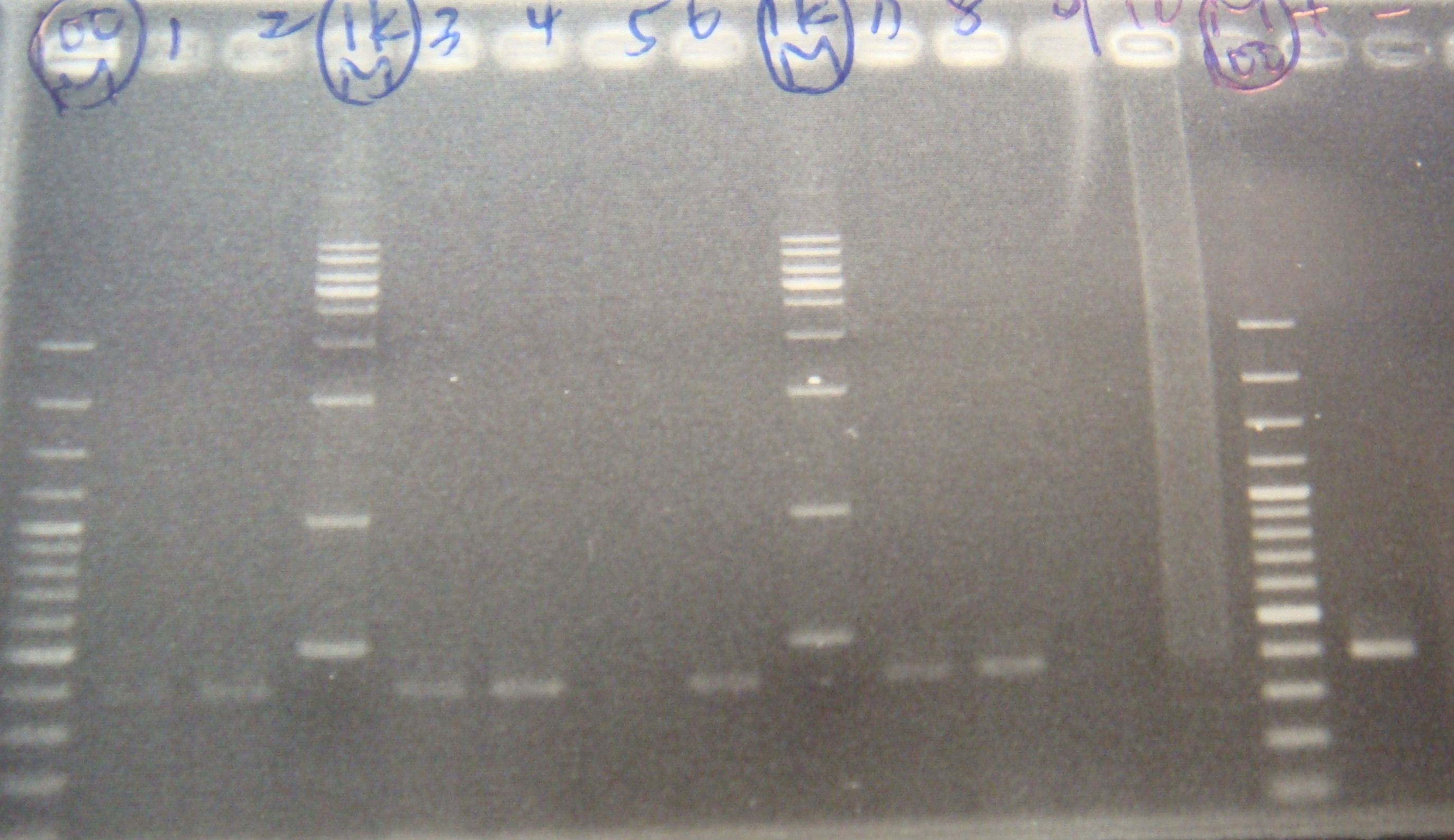 |
| Total: | 49μl | X3.8μl
|
| VR+VF | 2μl | 7.6μl
|
| dNTP | 2μl | 7.6μl
|
| 10XBuff. | 5μl | 17μl
|
| tag | 0.25μl | 0.95mu;l
|
| ddH2O | 39.75μl | 151.06μl
|
| PCR Protocol |
|
| 94
| 60s
|
| 94
| 15s
|
| 55
| 20s
|
| 72
| 90s
| 30 cycles
|
| 72
| 300s
|
|
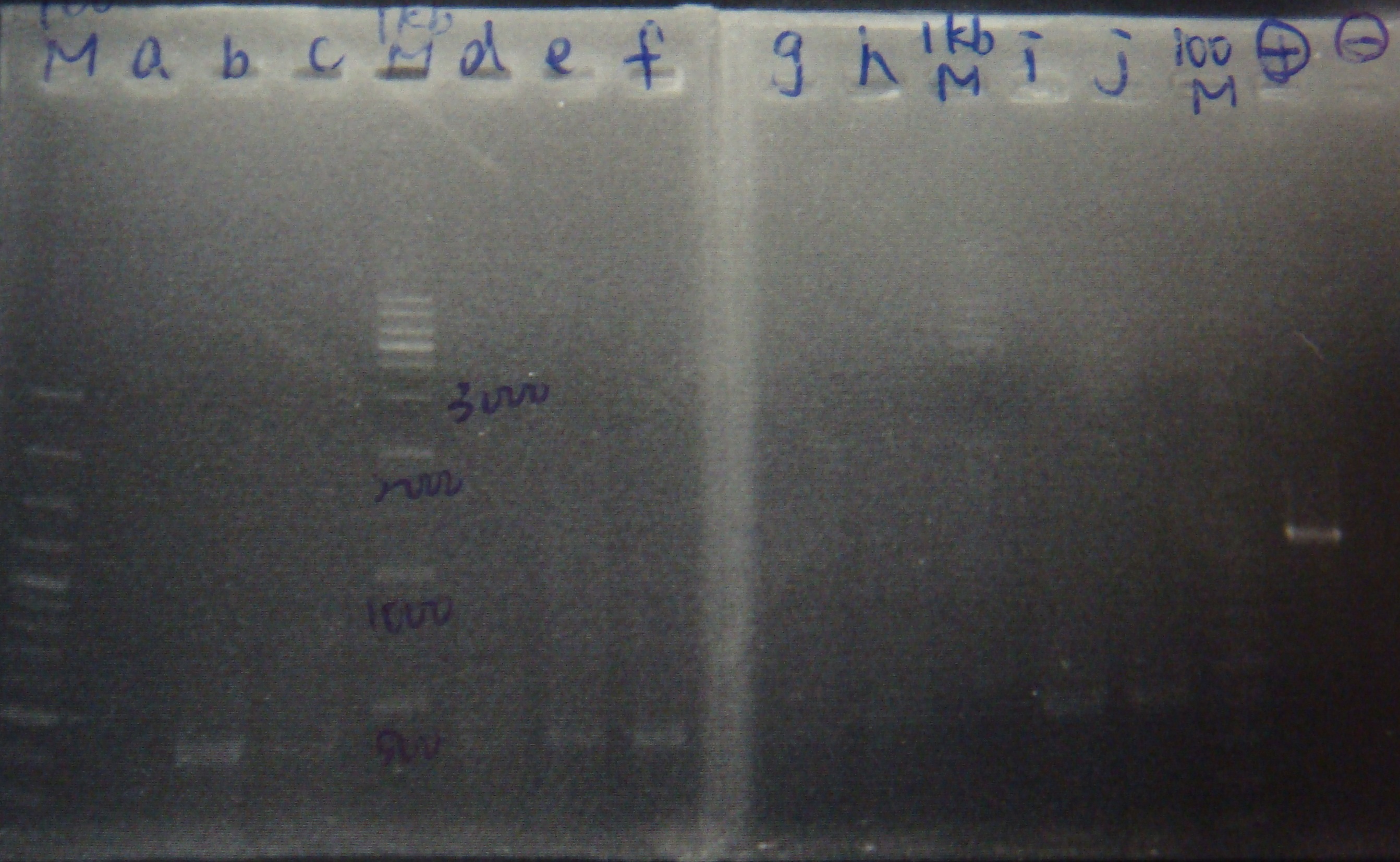 |
| Total: | 49μl | X3.8μl
|
| VR+VF | 2μl | 7.6μl
|
| dNTP | 2μl | 7.6μl
|
| 10XBuff. | 5μl | 17μl
|
| tag | 0.25μl | 0.95mu;l
|
| ddH2O | 39.75μl | 151.06μl
|
| PCR Protocol |
|
| 94
| 60s
|
| 94
| 15s
|
| 55
| 20s
|
| 72
| 180s
| 30 cycles
|
| 72
| 300s
|
|
- Ligate FRV with pLac again
- Transform
2010.10.10
 |
| Total: | 49μl | X3.2μl
|
| VR+VF | 2μl | 6.4μl
|
| dNTP | 2μl | 6.4μl
|
| 10XBuff. | 5μl | 15μl
|
| tag | 0.25μl | 0.8mu;l
|
| ddH2O | 39.75μl | 127.2μl
|
| PCR Protocol |
|
| 94
| 60s
|
| 94
| 15s
|
| 55
| 20s
|
| 72
| 180s
| 30 cycles
|
| 72
| 300s
|
|
length is correct!!!
- F colony liquid culture
- FRV liquid culture
2010.10.11
- FRV&PFRV plasmid extraction
- FRV cut gel
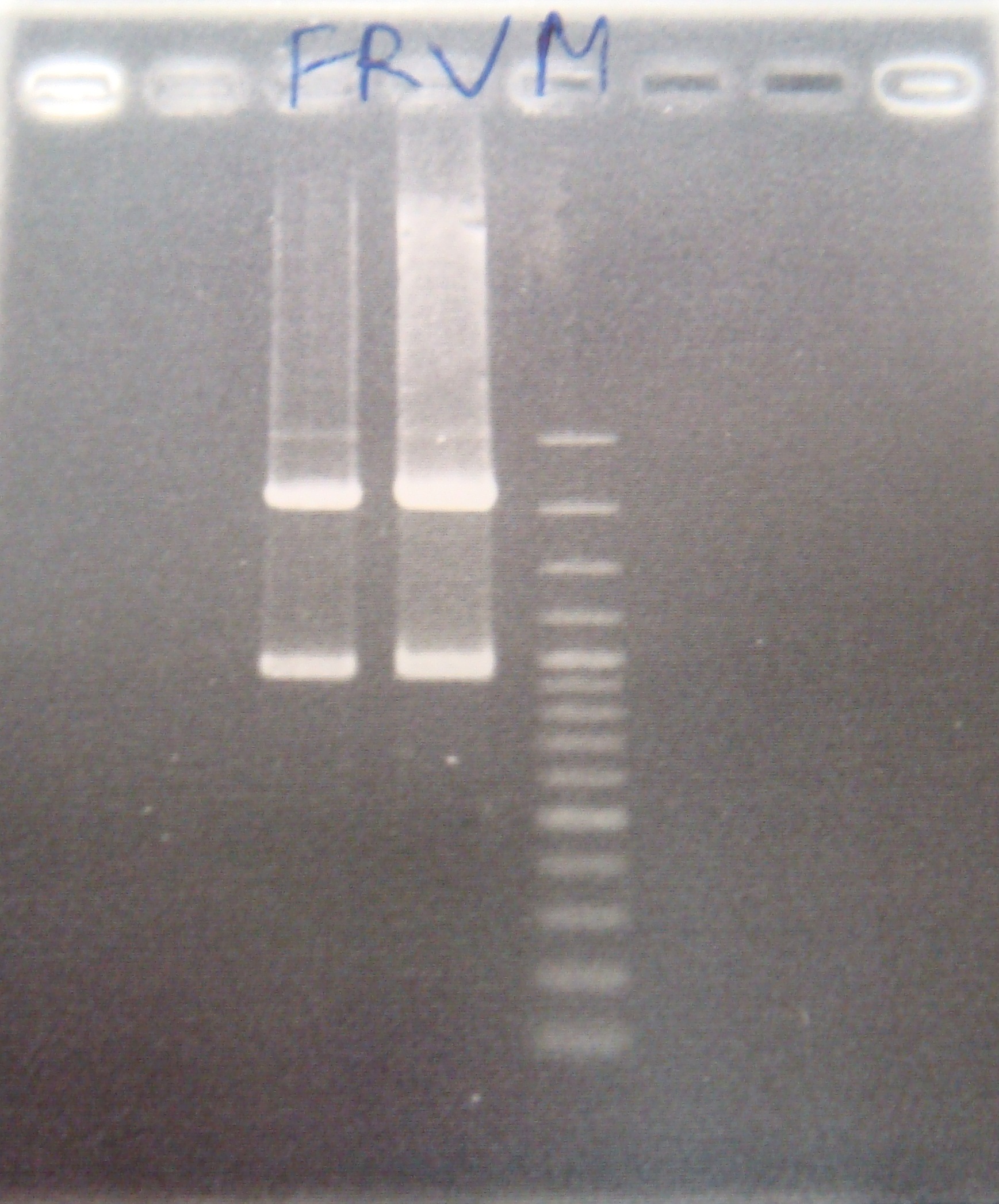
- Digest PFRV(XP)&FRV&pSB1C3
- Ligate PFRV with C3/ FRV with C3/ ribo with C3
- Transform
2010.10.12
- Ligate PFRV with C3/FRV with C3/ribo with C3 again
- Transform 17:25
2010.10.13
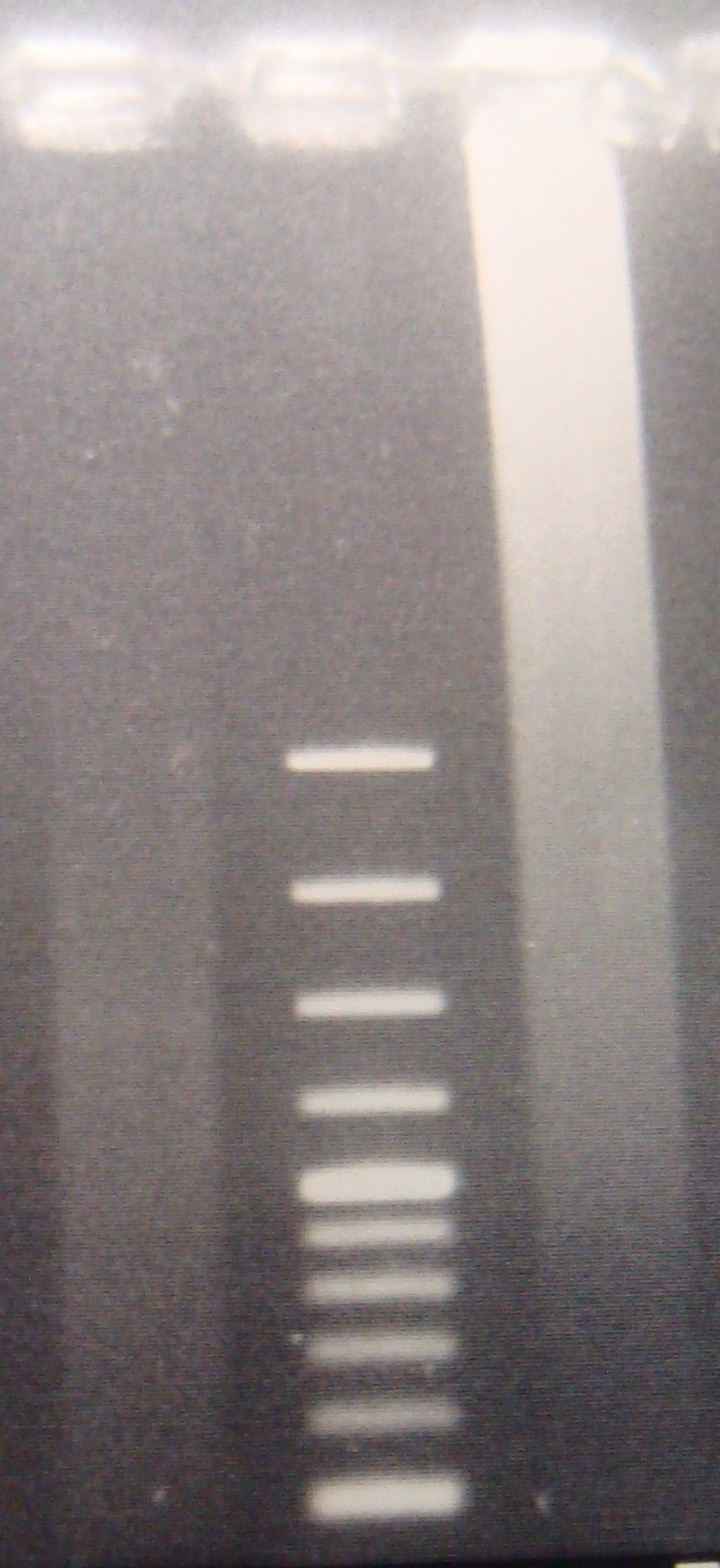 |
| Total: | 49μl | X1.7μl
|
| VR+VF | 2μl | 3.4μl
|
| dNTP | 2μl | 3.4μl
|
| 10XBuff. | 5μl | 8μl
|
| tag | 0.25μl | 0.425mu;l
|
| ddH2O | 39.75μl | 67.575μl
|
| PCR Protocol |
|
| 94
| 60s
|
| 94
| 15s
|
| 55
| 20s
|
| 72
| 90s
| 30 cycles
|
| 72
| 300s
|
|
- Ligate PFRV,FRV,ribo with C3
- Transform 00:50
- Digest PFRV(XP)&RV(XP) again overnight
2010.10.14
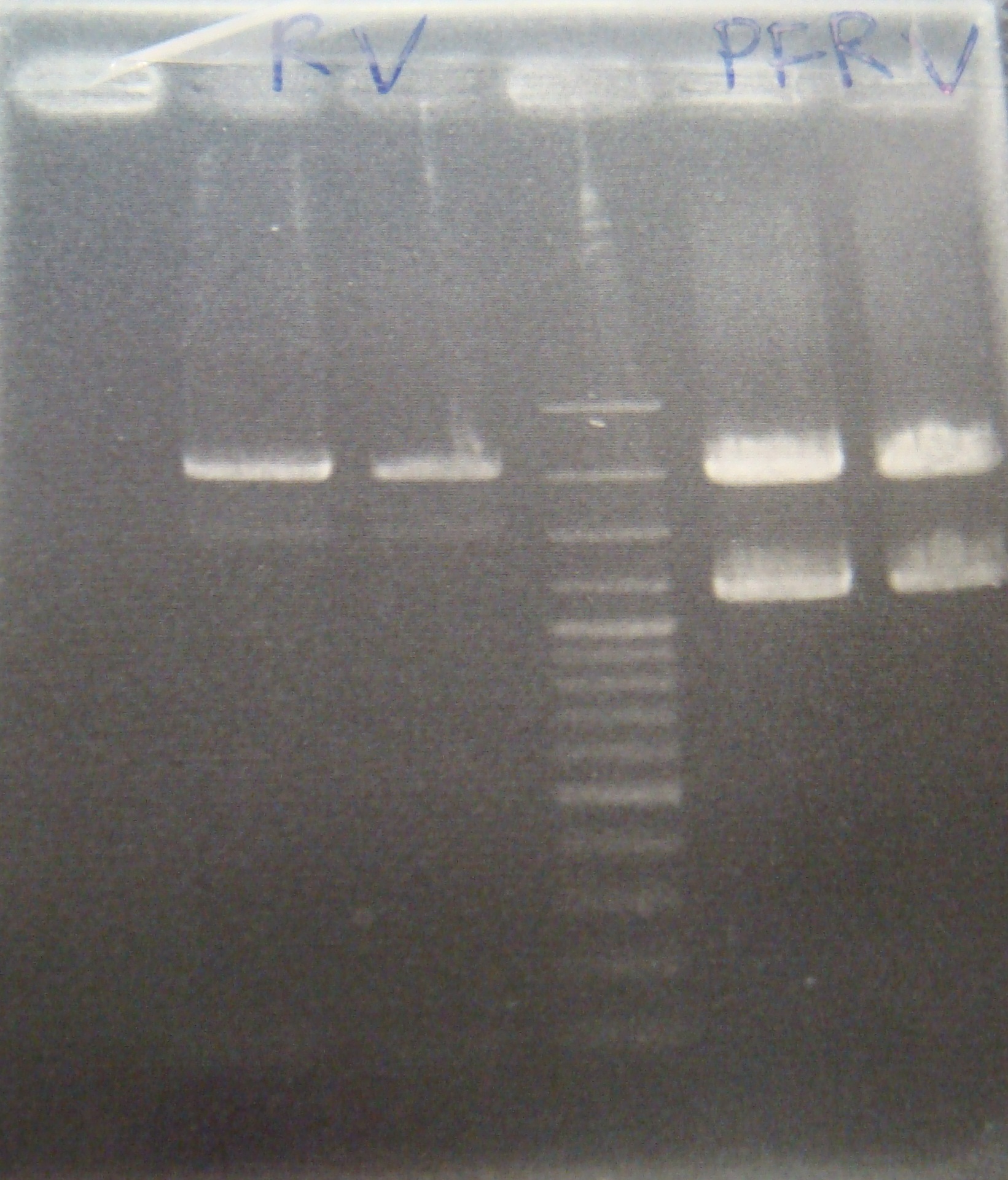
- Ligate RV,FRV,PFRV with C3 again
- Transform
2010.10.15
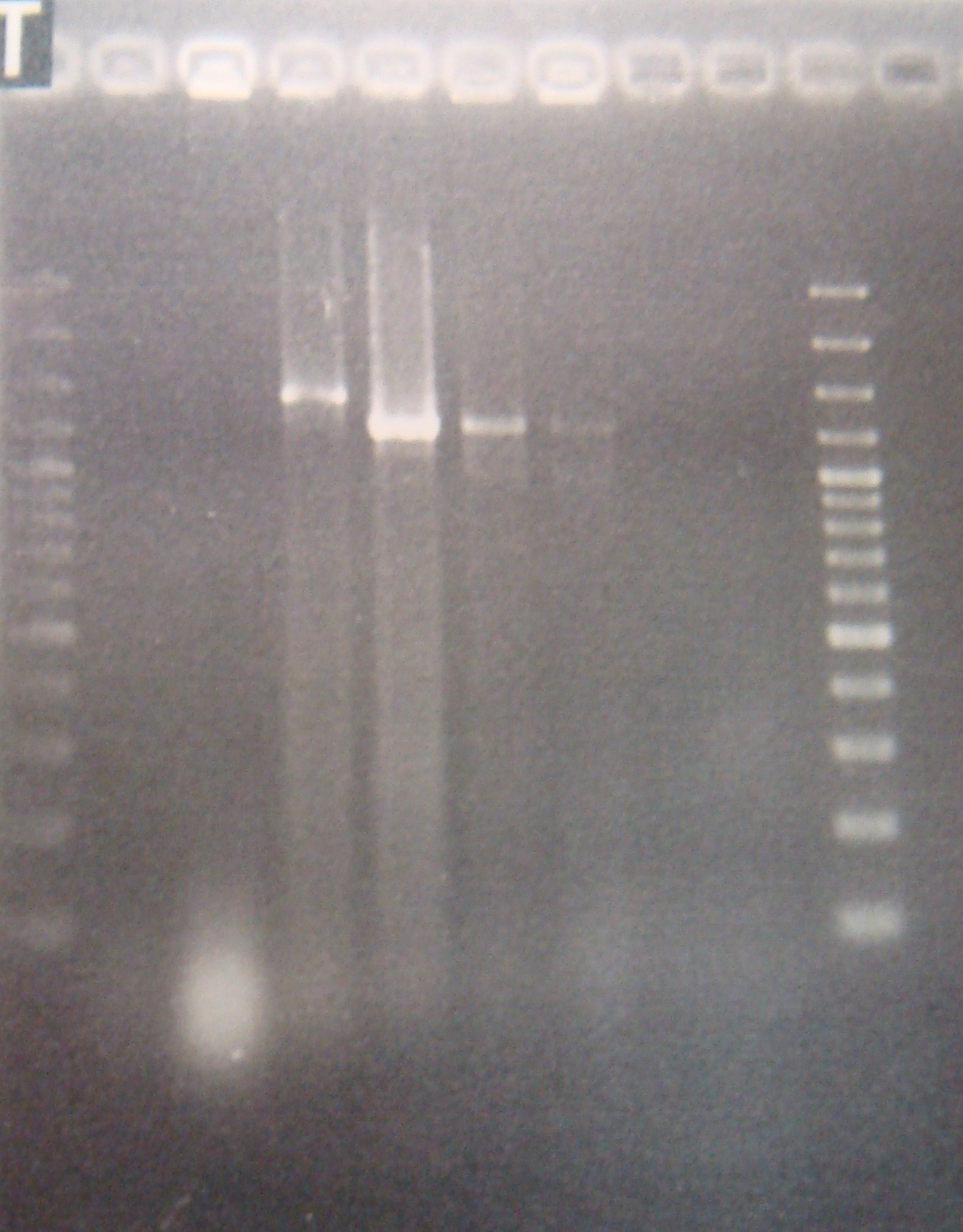 |
| Total: | 49μl | X2.5μl
|
| VR+VF | 2μl | 5μl
|
| dNTP | 2μl | 5μl
|
| 10XBuff. | 5μl | 12.5μl
|
| tag | 0.25μl | 0.625mu;l
|
| ddH2O | 39.75μl | 99.375μl
|
| PCR Protocol |
|
| 94
| 60s
|
| 94
| 15s
|
| 55
| 20s
|
| 72
| 90s
| 30 cycles
|
| 72
| 300s
|
|
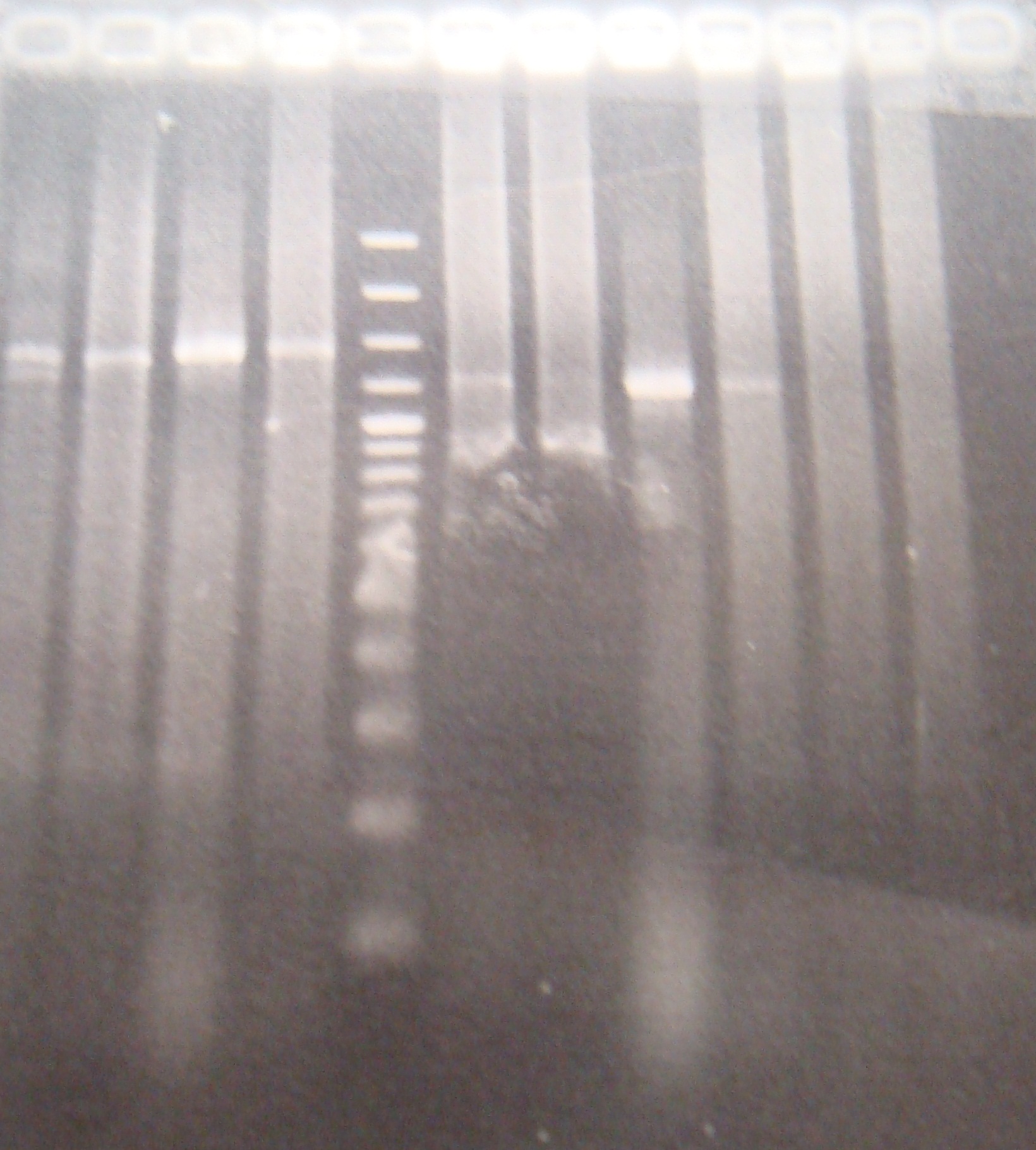 |
| Total: | 49μl | X2.5μl
|
| VR+VF | 2μl | 5μl
|
| dNTP | 2μl | 5μl
|
| 10XBuff. | 5μl | 12.5μl
|
| tag | 0.25μl | 0.625mu;l
|
| ddH2O | 39.75μl | 99.375μl
|
| PCR Protocol |
|
| 94
| 60s
|
| 94
| 15s
|
| 55
| 20s
|
| 72
| 90s
| 30 cycles
|
| 72
| 300s
|
|
FRC and PFRC are correct, but RC is still incorrect
- Ligate ribo with C3 again
- Transform
2010.10.16
- Theophylline solution
- Take 1.8012g Theophylline into 15ml DMSO;concentration is 0.67M
- put 15 μl into LB liquid, concentration is 2.5mM;
put 30 μl into LB liquid, concentration is 5mM
- Ligate ribo(xp) with C3 again
- Transform
2010.10.17
- transform second plate of PFRC into agar plate
positive:C3 30μl+theophylline 0.0027g
negative:C3
- PFRC&FRC plasmid extraction
2010.10.18
 |
| Total: | 49μl | X1.3μl
|
| VR+VF | 2μl | 2.6μl
|
| dNTP | 2μl | 2.6μl
|
| 10XBuff. | 5μl | 6.5μl
|
| tag | 0.25μl | 0.325mu;l
|
| ddH2O | 39.75μl | 51.675μl
|
| PCR Protocol |
|
| 94
| 60s
|
| 94
| 15s
|
| 55
| 20s
|
| 72
| 90s
| 30 cycles
|
| 72
| 300s
|
|
- Theophylline in DMSO
- add 1 ml DMSO + Theophylline into 4 ml LB
- Ligate ribo with C3 again
- Transform
- Digest ribo(XP)& C3(XP)
- ribo & C3 cut gel

2010.10.19
| | O.D/2 | O.D | add to liquid
|
| PFRC | 2.503 | 5.006 | 166.5
|
| LB | 0 | 0 |
|
- Because Theophylline entry into E. coli spend 6 hours at least, O.D= 0.0325
- Assay:add 80 μl PFRC liquid into LB
| | Theophylline concentration | Theophylline(0.67M) | PFRC liquid(μl) | Cm50(μl)
|
| A | 0 mM | 0 | 80.4 | 4
|
| B | 0.5 mM | 2.985 μl | 80.4 | 4
|
| C | 1 mM | 5.97 μl | 80.4 | 4
|
| D | 2 mM | 11.94 μl | 80.4 | 4
|
| E | 4 mM | 23.88 μl | 80.4 | 4
|
| F | 5 mM | 29.85 μpl | 80,4 | 4
|
 |
| Total: | 49μl | X2.1μl
|
| VR+VF | 2μl | 4.2μl
|
| dNTP | 2μl | 4.2μl
|
| 10XBuff. | 5μl | 10.5μl
|
| tag | 0.25μl | 0.525mu;l
|
| ddH2O | 39.75μl | 83.475μl
|
| PCR Protocol |
|
| 94
| 60s
|
| 94
| 15s
|
| 55
| 20s
|
| 72
| 30s
| 30 cycles
|
| 72
| 300s
|
|
- Ligate ribo with C3 again
- Transform
2010.10.20
| | Theophylline concentration | Theophylline(0.67M) | PFRC liquid(μl) | Cm50(μl)
|
| O | 0 mM | 0 | 130 | 4
|
| A | 0.1 mM | 0.597 μl | 130 | 4
|
| B | 0.05 mM | 1.4925 μl | 130 | 4
|
| C | 0.5 mM | 2.985 μl | 130 | 4
|
| D | 1 mM | 5.97 μl | 130 | 4
|
| E | 2 mM | 11.94 μl | 130 | 4
|
| F | 4 mM | 23.88 μpl | 130 | 4
|
| G | 8 mM | 47.76 μl | 130 | 4
|
culture for 2 hours
2010.10.21
| | Theophylline concentration | Theophylline(0.67M) | PFRC liquid(μl) | Cm50(μl)
|
| O | 0 μM | 0 | 85.7 | 4
|
| A | 50 μM | 0.2985 μl | 85.7 | 4
|
| B | 100 μM | 0.597 μl | 85.7 | 4
|
| C | 200 μM | 1.194 μl | 85.7 | 4
|
| D | 500 μM | 2.985 μl | 85.7 | 4
|
| E | 1000 μM | 5.97 μl | 85.7 | 4
|
| F | 2000 μM | 11.94 μl | 85.7 | 4
|
| G | 4000 μM | 23.88 μl | 85.7 | 4
|
| H | 8000 μM | 47.76 μl | 85.7 | 4
|
| I | 10000 μM | 59.7 μl | 85.7 | 4
|
| J | 20000 μM | 119.4 μl | 85.7 | 4
|
| K | 40000 μM | 238.8 μl | 85.7 | 4
|
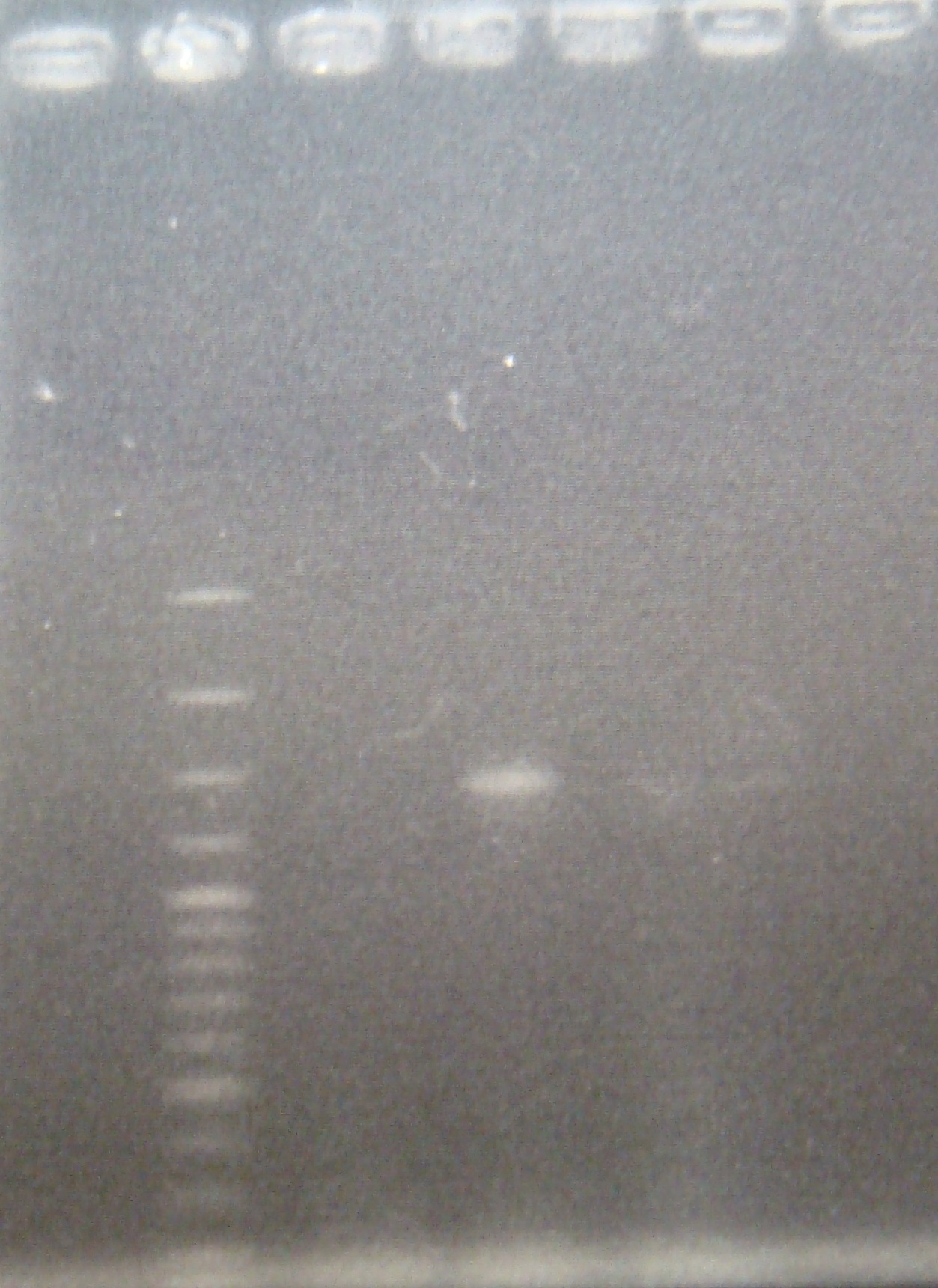 |
| Total: | 49μl | X2.5μl
|
| VR+VF | 2μl | 5μl
|
| dNTP | 2μl | 5μl
|
| 10XBuff. | 5μl | 12.5μl
|
| tag | 0.25μl | 1.25mu;l
|
| ddH2O | 39.75μl | 99.375μl
|
| PCR Protocol |
|
| 94
| 60s
|
| 94
| 15s
|
| 55
| 20s
|
| 72
| 30s
| 30 cycles
|
| 72
| 300s
|
|
2010.10.22
- Digest C3 from PFRC plasmid
- Ligate ribo with C3
- Transform
- Theophylline solution; concentration is 0.1M
- Assay
| | Theophylline concentration | Theophylline(0.1M) | PFRC liquid(μl) | Cm50(μl)
|
| O | 0 μM | 0 | 85.7 | 4
|
| A | 10 μM | 0.4 μl | 117.7 | 4
|
| B | 50 μM | 2 μl | 117.7 | 4
|
| C | 100 μM | 4 μl | 117.7 | 4
|
| D | 200 μM | 8 μl | 117.7 | 4
|
| E | 500 μM | 20 μl | 117.7 | 4
|
| F | 1000 μM | 40 μl | 117.7 | 4
|
| G | 2000 μM | 80 μl | 117.7 | 4
|
| H | 4000 μM | 160 μl | 117.7 | 4
|
| I | 8000 μM | 320 μl | 117.7 | 4
|
| J | 10000 μM | 400 μl | 117.7 | 4
|
| K | 20000 μM | 800 μl | 117.7 | 4
|
| | Theophylline concentration | Theophylline(0.1M) | PFRC liquid(μl) | Cm50(μl)
|
| O | 0 μM | 0 | 261 | 4
|
| A | 10 μM | 0.4 μl | 261 | 4
|
| B | 50 μM | 2 μl | 261 | 4
|
| C | 100 μM | 4 μl | 261 | 4
|
| D | 200 μM | 8 μl | 261 | 4
|
| E | 500 μM | 20 μl | 261 | 4
|
| F | 1000 μM | 40 μpl | 261 | 4
|
| G | 2000 μM | 80 μl | 261 | 4
|
| H | 4000 μM | 160 μl | 261 | 4
|
| I | 80000 μM | 320 μl | 261 | 4
|
| J | 10000 μM | 400 μl | 261 | 4
|
| K | 20000 μM | 800 μl | 261 | 4
|
| NT | 100 μM | 4 μl | 261 | 4
|
| N | 0 μM | 0 μl | 261 | 4
|
NT: FRC(without promoter)+Theophylline
N: FRC(without promoter)
2010.10.24
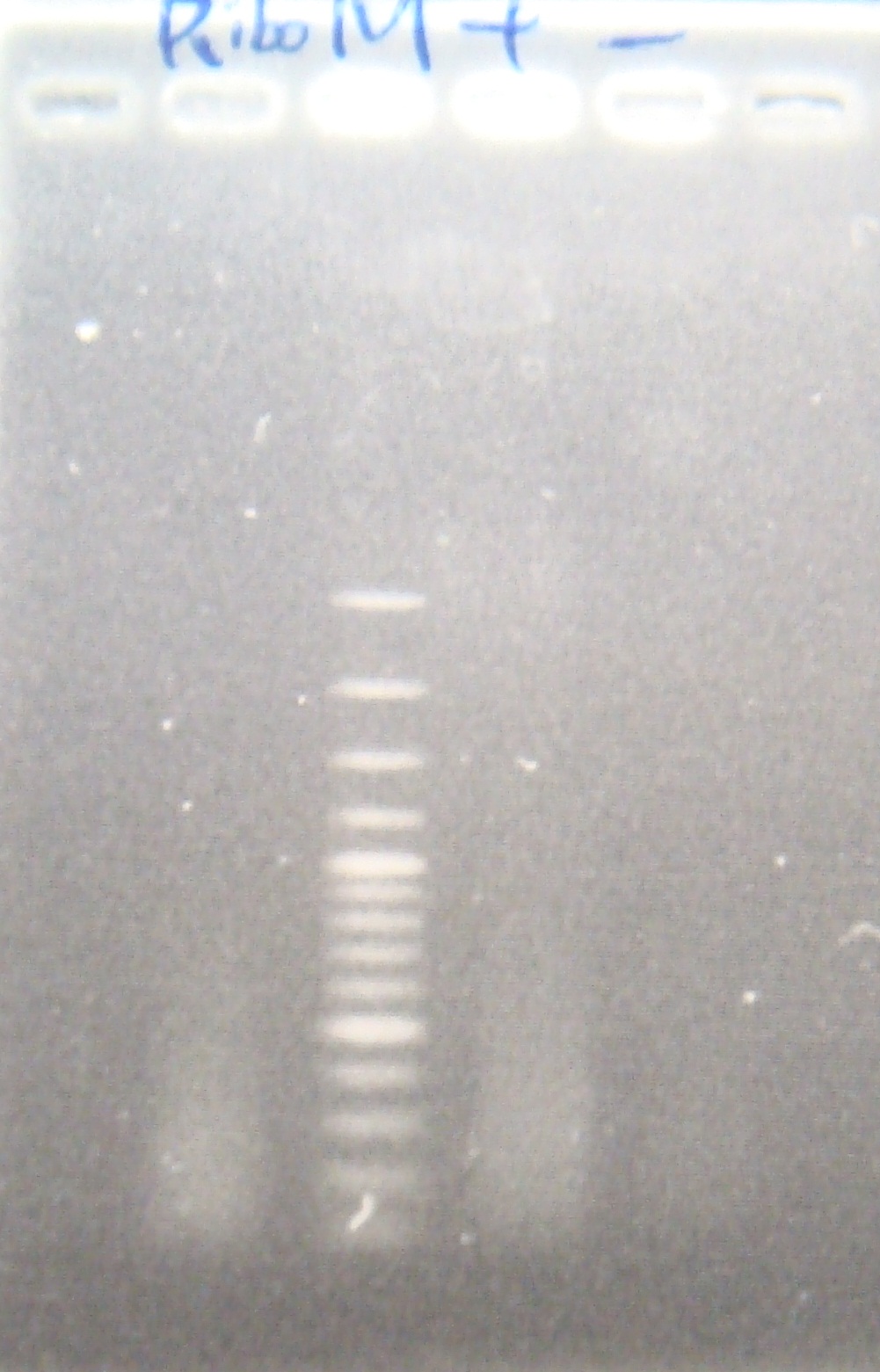 |
| Total: | 49μl | X0.9μl
|
| VR+VF | 2μl | 1.8μl
|
| dNTP | 2μl | 1.8μl
|
| 10XBuff. | 5μl | 4.5μl
|
| tag | 0.25μl | 0.225mu;l
|
| ddH2O | 39.75μl | 35.775μl
|
| PCR Protocol |
|
| 94
| 60s
|
| 94
| 15s
|
| 55
| 20s
|
| 72
| 30s
| 30 cycles
|
| 72
| 300s
|
|
- ribo liquid culture
- colony PCR
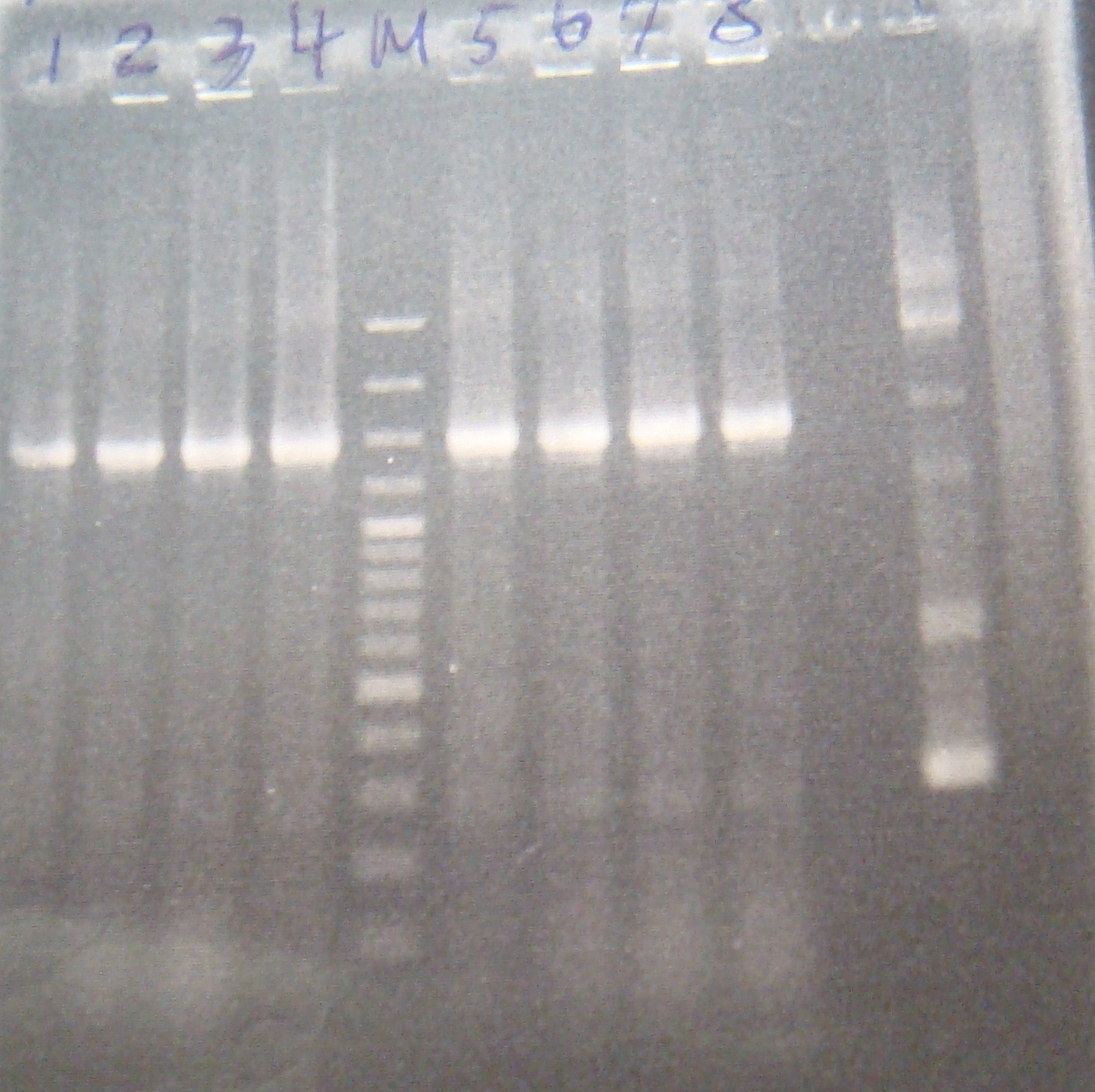 |
| Total: | 49μl | X1.6μl
|
| VR+VF | 2μl | 3.2μl
|
| dNTP | 2μl | 3.2μl
|
| 10XBuff. | 5μl | 8μl
|
| tag | 0.25μl | 0.8mu;l
|
| ddH2O | 39.75μl | 63μl
|
| PCR Protocol |
|
| 94
| 60s
|
| 94
| 15s
|
| 55
| 20s
|
| 72
| 30s
| 30 cycles
|
| 72
| 300s
|
|
| | Theophylline concentration | Theophylline(0.1M) | LB(μl) | PFRC liquid(μl) | Cm50(μl)
|
| O | 0 μM | 0 | 325 | 325 | 4
|
| A | 10 μM | 0.4 μl | 325 | 325 | 4
|
| B | 50 μM | 2 μl | 327 | 325 | 4
|
| C | 100 μM | 4 μl | 329 | 325 | 4
|
| D | 200 μM | 8 μl | 333 | 325 | 4
|
| E | 500 μM | 20 μl | 345 | 325 | 4
|
| F | 1000 μM | 40 μpl | 365 | 325 | 4
|
| G | 2000 μM | 80 μl | 405 | 325 | 4
|
| H | 4000 μM | 160 μl | 485 | 325 | 4
|
| I | 8000 μM | 320 μl | 645 | 325 | 4
|
| J | 10000 μM | 400 μl | 725 | 325 | 4
|
| K | 20000 μM | 800 μl | 1125 | 325 | 4
|
| NT | 100 μM | 4 μl | 504 | 500(FRC) | 4
|
| N | 0 μM | 0 μl | 500 | 500(FRC) | 4
|
- Digest RV(XP)& FRC(XP)
- Ligate ribo with C3
- transform
2010.10.25
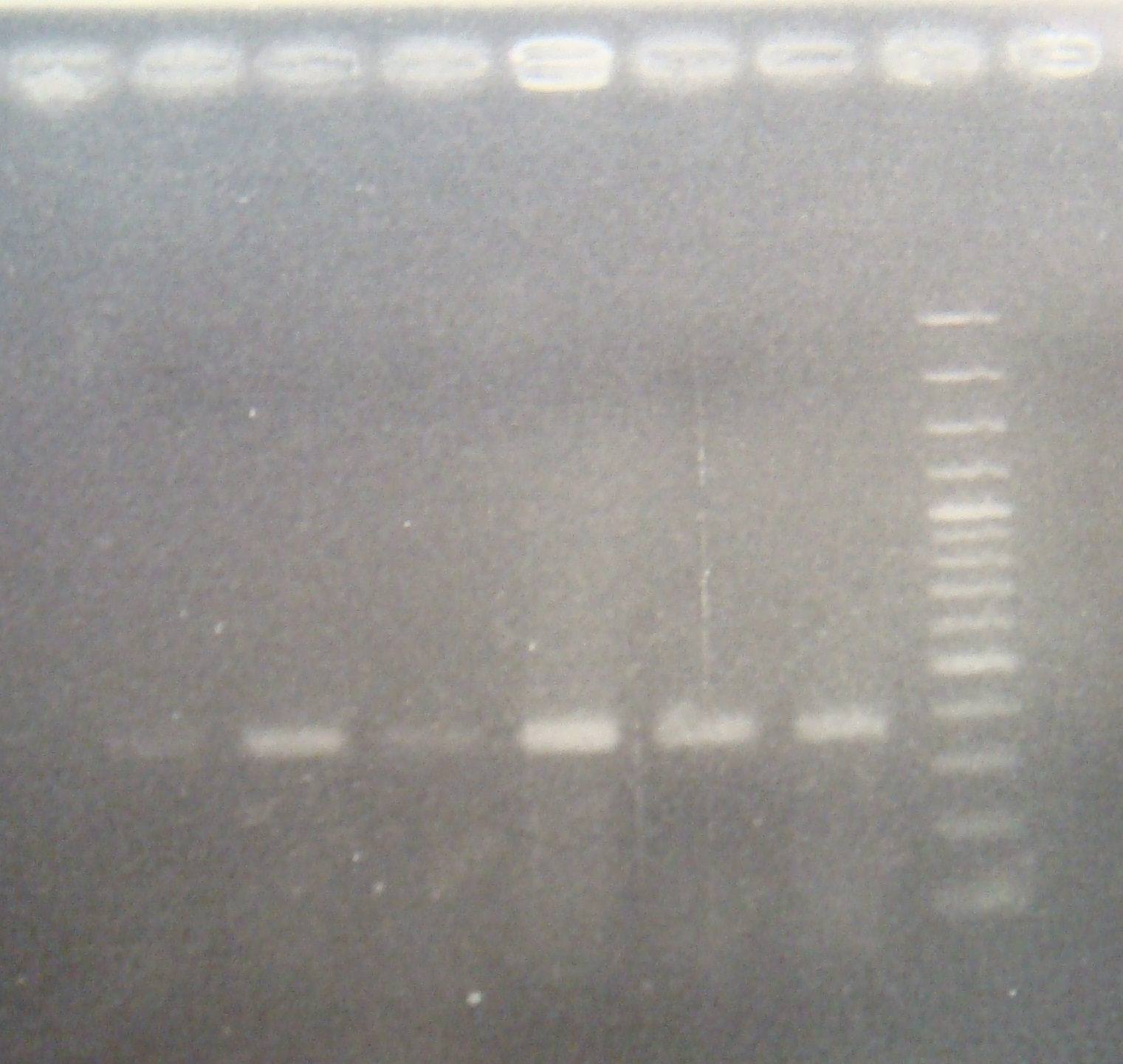 |
| Total: | 49μl | X2.4μl
|
| VR+VF | 2μl | 4.8μl
|
| dNTP | 2μl | 4.8μl
|
| 10XBuff. | 5μl | 12μl
|
| tag | 0.25μl | 0.6mu;l
|
| ddH2O | 39.75μl | 95.4μl
|
| PCR Protocol |
|
| 94
| 60s
|
| 94
| 15s
|
| 55
| 20s
|
| 72
| 30s
| 30 cycles
|
| 72
| 300s
|
|
- FRC&PFRC&RC liquid culture
| | Theophylline concentration | Theophylline(0.1M) | LB(μl) | PFRC liquid(μl) | Cm50(μl)
|
| O | 0 μM | 0 | 151 | 147 | 4
|
| A | 50 μM | 2 μl | 153 | 147 | 4
|
| B | 500 μM | 20 μl | 171 | 147 | 4
|
| C | 1000 μM | 40 μl | 191 | 147 | 4
|
| D | 4000 μM | 160 μl | 311 | 147 | 4
|
| E | 10000 μM | 400 μl | 551 | 147 | 4
|
| NT | 100 μM | 4 μl | 158 | 150(FRC) | 4
|
| N | 0 μM | 0 μl | 154 | 150(FRC) | 4
|
2010.10.26
| | Theophylline concentration | Theophylline(0.1M) | LB(μl) | PFRC liquid(μl) | Cm50(μl)
|
| O | 0 μM | 0 | 151 | 147 | 4
|
| A | 50 μM | 2 μl | 153 | 147 | 4
|
| B | 500 μM | 20 μl | 171 | 147 | 4
|
| C | 1000 μM | 40 μl | 191 | 147 | 4
|
| D | 4000 μM | 160 μl | 311 | 147 | 4
|
| E | 10000 μM | 400 μl | 551 | 147 | 4
|
| NT | 100 μM | 4 μl | 158 | 150(FRC) | 4
|
| N | 0 μM | 0 μl | 154 | 150(FRC) | 4
|
|
|
|
|
|
|
|
|
|
|
|
|
|
 "
"