Team:Kyoto/Notebook2
From 2010.igem.org
Notebook2
Measurement of R0011
Wednesday, August 11 By: Wataru
Overnight culture
| Name | Strain | Medium | Inducer(IPTG) | Incubation |
|---|---|---|---|---|
| ML- | KRX | LB (Kan+), 3mL | 0mM | At 37℃ 20:20- (Overnight) |
| ML+ | KRX | 1mM | ||
| MS- | KRX | 0mM | ||
| MS+ | KRX | 1mM | ||
| KRX | KRX | SOC, 3mL | 0mM |
Thursday, August 12 By: Tomonori, Takuya O.
Culture
We stopped overnight cultures at 11:10 and took out 30µL culture from each test tube, and added it to 3mL fresh medium which is same for overnight culture. They are grown at 37℃ from 11:10-14:10. After 3-hours culture, OD600 of each culture were measured. Because we found that the 3-hours culture of the sample KRX had not grown, we use the overnight culture of the sample KRX in later procedures. We took out 1mL medium from test tubes and put them into 1.5mL tubes and freeze them. The data is shown in table below.
Measure
We melt samples and centrifuge them at 14,000rpm, at 4℃, for 2min. Discard the supernatant and 150µL cel lysis reagent to each pellet. These pellets were dissolved by vortex for 2min and centrifuged for 2min. From each tubes, 100µL of the supernatant was took out and added to each well of the plate for the plate reader. 100µL cell lysis reagent was also added to them. We measured GFP fluorescence in each sample (Ex/Em = 485/535nm, 1sec). The data is shown in table below. We calculated the promoter activity and the results are shown in table below.
| Name | Optical Density (600nm) | Fluorescence (485/535nm) | Calculation 1 | Calculation 2 | Calculation 3 | Promoter Activity |
|---|---|---|---|---|---|---|
| ML- | 0.531 | 4881 | 4697 | 8.85 × 103 | 6.40 × 103 | 8.65 × 10-2 |
| ML+ | 0.546 | 91549 | 91365 | 1.67 × 105 | 1.65 × 105 | 2.04 |
| MS- | 0.645 | 49469 | 49285 | 7.64 × 104 | 7.40 × 104 | 1 (0mM IPTG) |
| MS+ | 0.623 | 52017 | 51833 | 8.32 × 104 | 8.08 × 104 | 1 (1mM IPTG) |
| KRX | 1.413 | 3644 | 3460 | 2.449 × 103 | - | - |
| Cell Lysis Reagent | - | 184 | - | - | - | - |
- Calculation 1: Fluorescence - FluorescenceCell Lysis Reagent
- Calculation 2: Calculation 1 / Optical Density (600nm)
- Calculation 3: Calculation 2 - Calculation 2KRX
- Promoter Activity: Calculation 3ML- / Calculation 3MS-, Calculation 3ML+ / Calculation 3MS+
Friday, August 20 By: Tomonori
Make M9 Medium
M9 salts were added to 1l MilliQ and sterilized by autocrave. M9 medium was stored in a fridge.
Monday, August 23 By: Tasuku, Takuya O.
Make Supplemented M9 Medium
We tried to dissolve CaCl2 0.1M (final concentration) in 1l M9 medium, but we failed because CaCl2 was too much.
Tuesday, August 24 By: Tasuku, Takuya O.
Make Supplemented M9 Medium
M9 salts were added to 1l MilliQ and sterilized by autocrave. We changed amount of CaCl2 from 0.1M (final concentration) to 0.01mM (final concentration), and we could make supplemented M9 medium.
Overnight culture
The ML, i.e., E.coli KRX transformed with <partinfo>K358000</partinfo> was grown in the supplemented M9 medium including 1mM IPTG for a night (16h).
Wednesday, August 25 By: Tasuku, Takuya O.
Culture
OD600 of the overnight culture was measured. The overnight culture was diluted in fresh supplemented M9 medium including 7 different IPTG concentration and OD600 of each medium was measured every 4 hours. The samples to measure fluorescence of GFP were also collected (To know more detail procedures, see Protocols).
| 0.00mM | 0.01 | 0.03 | 0.05 | 0.07 | 0.10 | 1.00 | |
|---|---|---|---|---|---|---|---|
| 0h | -0.003 | 0.014 | 0.000 | -0.008 | - | - | - |
| 4h | 0.001 | 0.100 | 0.033 | 0.062 | - | - | - |
| 6h | - | - | - | - | 0.118 | 0.187 | 0.178 |
| 8h | 0.227 | 0.402 | 0.363 | 0.346 | 0.360 | 0.479 | 0.517 |
| 16 | 1.117 | 1.142 | 1.131 | 1.237 | 1.211 | 1.202 | 1.156 |
| 20h | 1.107 | 1.118 | 1.084 | 1.178 | 1.177 | 1.178 | 1.131 |
- The OD600 of medium including 0.07mM, 0.10mM, and 1mM IPTG were not measured at 4h, but at 6h.
- Measurement at 16h and 20h was conducted the day after.
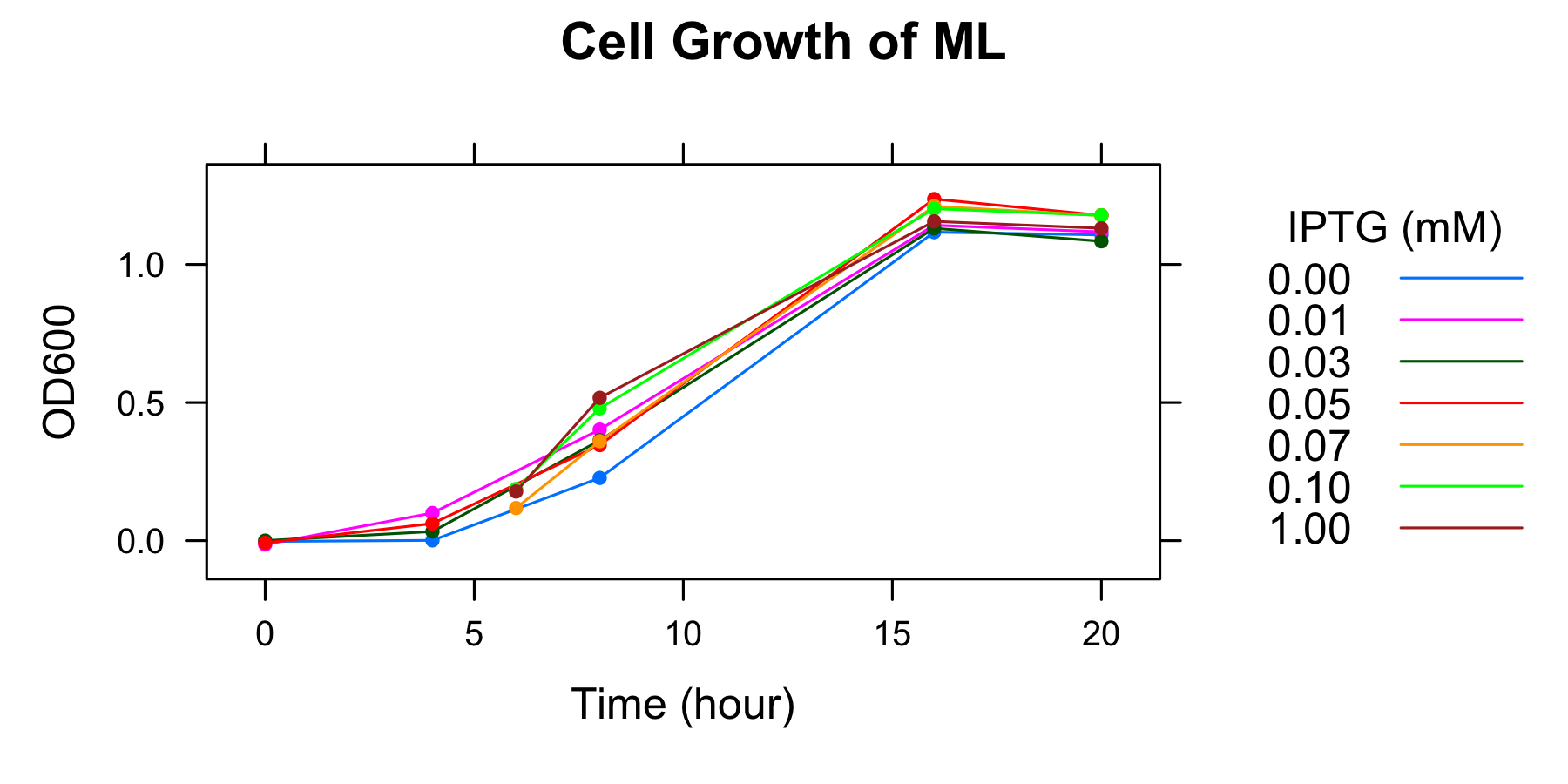 We found that the number of the cell was in steady state at 16h and after.
We found that the number of the cell was in steady state at 16h and after.
Overnight Culture
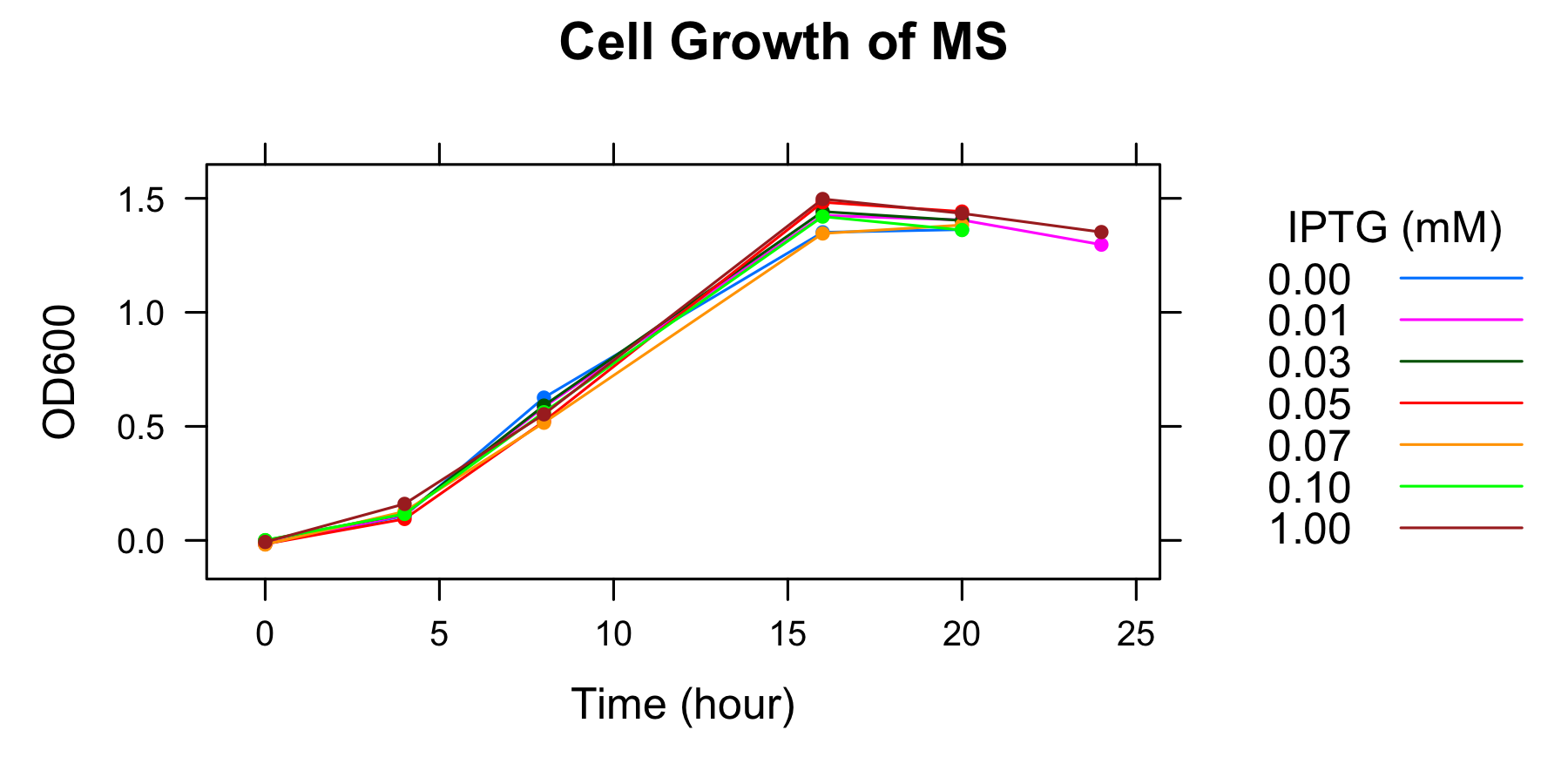
The MS, i.e., E.coli KRX transformed with <partinfo>I20260</partinfo> was grown in supplemented M9 medium including 1mM IPTG for a night for 16 hours.
Thursday, August 26 By: Tasuku, Takuya O.
Culture
OD600 of the overnight culture was measured. The overnight culture was diluted in fresh supplemented M9 medium including 7 different IPTG concentration and OD600 of each medium was measured every 4 hours. The samples to measure fluorescence of GFP were also collected (To know more detail procedures, see Protocols).
| Time | 0.00mM | 0.01 | 0.03 | 0.05 | 0.07 | 0.10 | 1.00 |
|---|---|---|---|---|---|---|---|
| 0h | -0.016 | -0.015 | 0.000 | -0.016 | -0.018 | 0.001 | -0.007 |
| 4 | 0.108 | 0.112 | 0.116 | 0.094 | 0.127 | 0.116 | 0.160 |
| 8 | 0.626 | 0.585 | 0.591 | 0.522 | 0.516 | 0.562 | 0.553 |
| 20 | 1.363 | 1.405 | 1.403 | 1.442 | 1.383 | 1.361 | 1.434 |
| 24 | - | 1.297 | - | - | - | - | 1.352 |
- Measurement at 16h, 20h, and 24h was conducted the day after.
Measure OD600 of the culture of ML and collect samples
Friday, August 27 By: Tasuku, Takuya O.
Measure OD600 of the culture of ML and collect samples
Make the plate of E.coli C2=
The E.coli competent cell, C2, was diluted to three different concentration, that is 10-3, 10-5, 10<sup-6</sup>. The 200µL of each culture was plated without antibiotics.
Monday, August 30 By: Tomonori, Tasuku
Overnight culture
A colony of each plate made on Friday was picked out and put into the test tube including 5mL supplemented M9 medium without antibiotics. They were grown at 37℃ for a night (16 hours).
Tuesday, August 31 By: Tomonori, Tasuku, Kazuya
Measure OD600 of the overnight culture
OD600 of the overnight culture was measured. We found that E.coli C2 had not grown.
| Name | OD600 |
|---|---|
| C2 (diluted to 10-3) | 0.034 |
| C3 (diluted to 10-5) | 0.062 |
| C4 (diluted to 10-6) | 0.040 |
Because we noticed that the color of the three overnight cultures were strange, we suspected that other bacterium had grwon in these culture.
Culture E.coli C2 wighout antibiotics again
A colony of paltes were picked out and put into the test tube including 5mL supplemented M9 medium wighout antibiotics. They were grown at 37℃ for 11 hours. OD600 of the culture was measured. As the previous experiment, C2 did not grow.
| Name | OD600 |
|---|---|
| C2 (diluted to 10-3) | 0.031 |
| C3 (diluted to 10-5) | 0.042 |
| C4 (diluted to 10-6) | 0.091 |
Overnight culture E.coli C2 in new medium
The supllemented M9 medium wighout antibiotics was made again. E.coli C2 (diluted to 10-3) was grown for a night (16 hours).
Measure the fluorescence of GFP of the ML and the MS
The fluorescence of GFP of the ML and the MS was measured (Ex/Em = 485/535 nm, 1sec).
| Time | 0.00mM | 0.01 | 0.03 | 0.05 | 0.07 | 0.10 | 1.00 |
|---|
- Back corrected.
| Time | 0.00mM | 0.01 | 0.03 | 0.05 | 0.07 | 0.10 | 1.00 |
|---|---|---|---|---|---|---|---|
| 0h | 3 | -13 | -11 | 16 | 56 | 32 | -14 |
| 4 | 17638 | 7790 | 13413 | 8056 | 19632 | 8090 | 8800 |
| 16 | 6504 | 7556 | 6159 | 7650 | 4228 | 4240 | 10662 |
| 20 | 7835 | 11638 | 9070 | 15135 | 15383 | 8681 | 18276 |
| 24 | - | 22526 | - | - | - | - | 34230 |
- Back corrected.
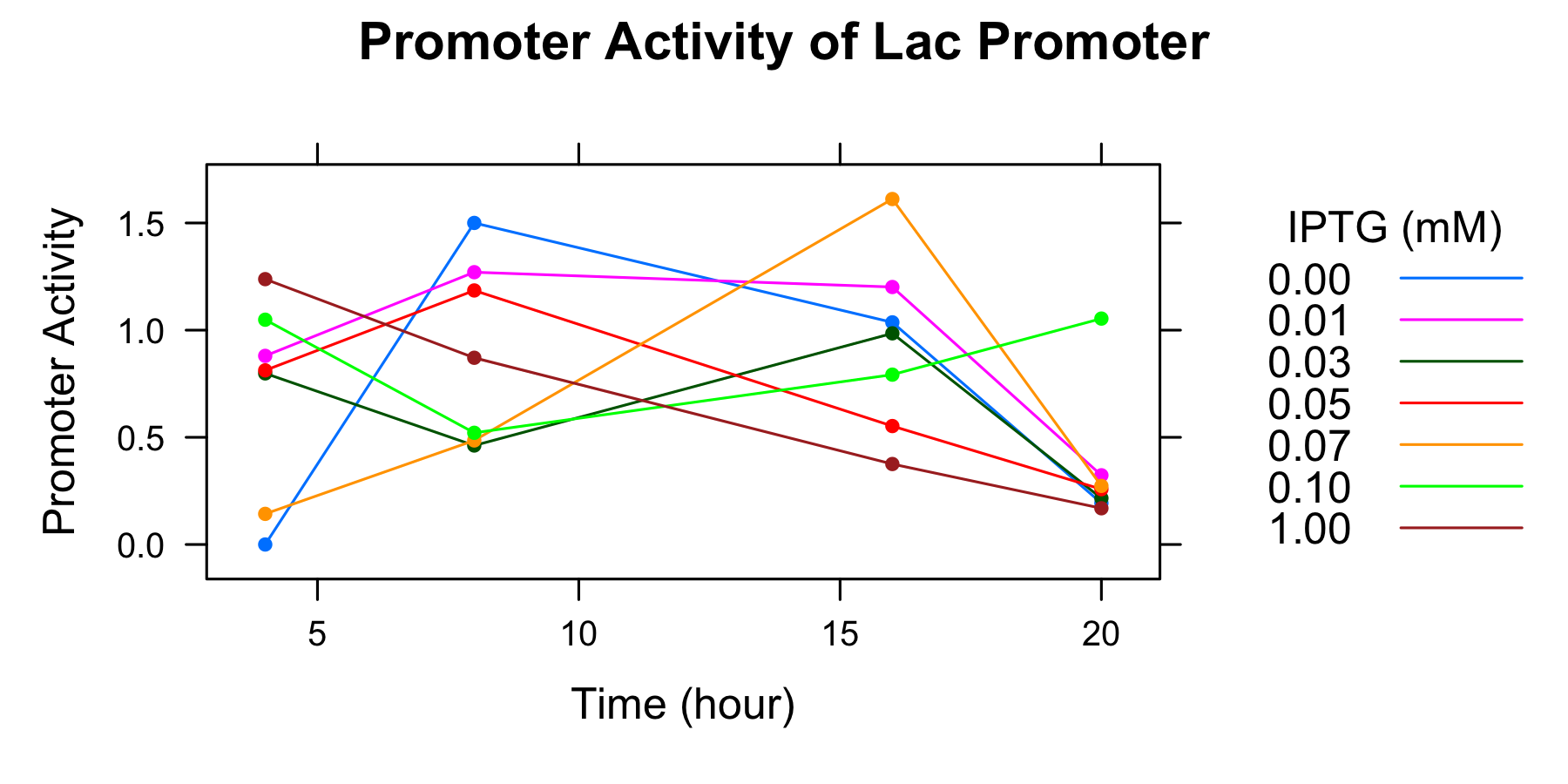
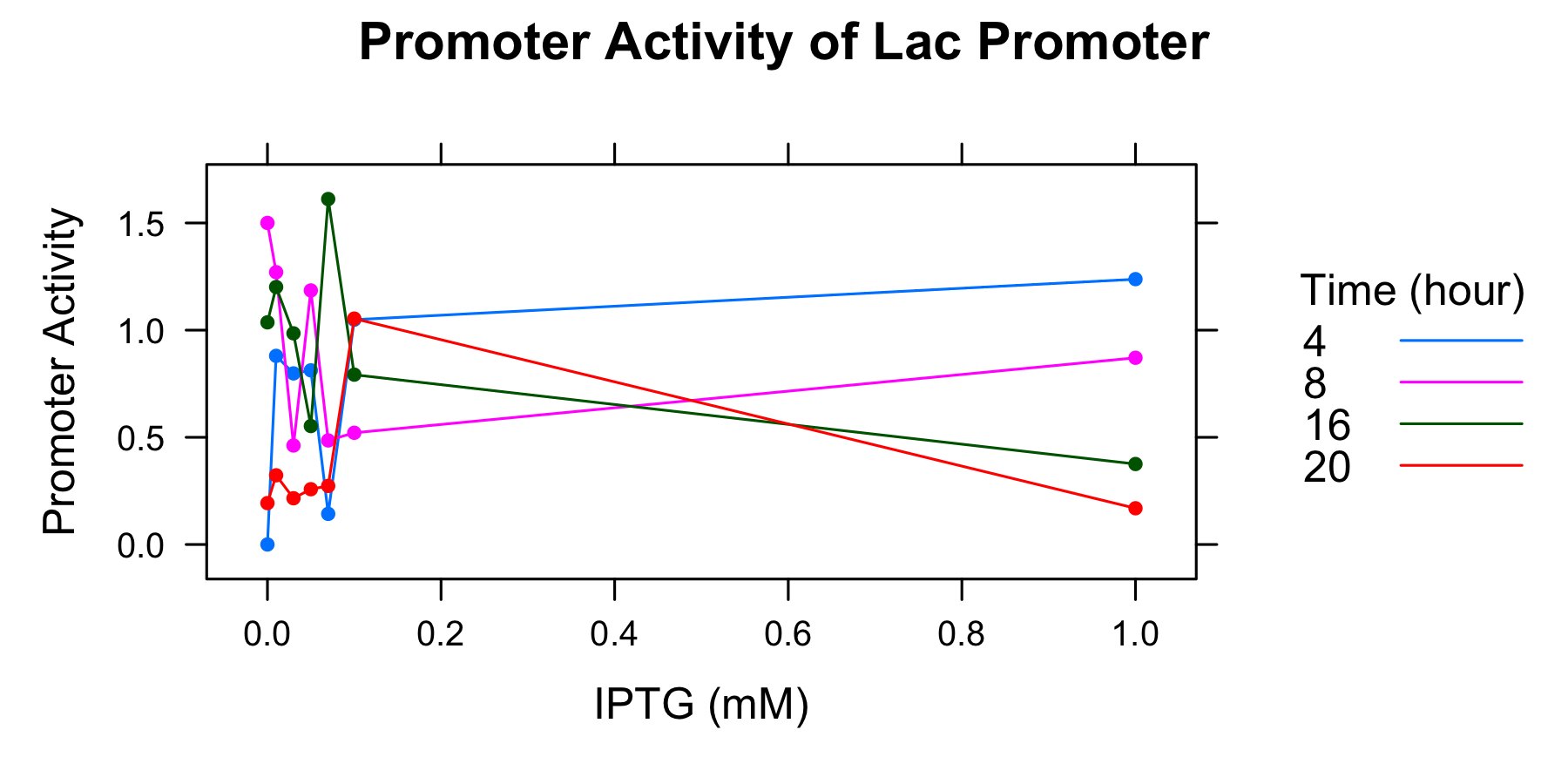
Because amounts of samples collected at 0h are very little and the data indicates that is not enough to measure OD600 and fluorescence of GFP correctoly, we decide to measure OD600 after 4 hours only. We calculated the promoter activity of the lactose promoter.
| Time | 0.00mM | 0.01 | 0.03 | 0.05 | 0.07 | 0.10 | 1.00 |
|---|---|---|---|---|---|---|---|
| 0h | - | - | - | - | - | - | - |
| 4 | 0.000000 | 0.880041 | 0.798267 | 0.812454 | 0.142867 | 1.048718 | 1.237998 |
| 8 | 1.500221 | 1.270405 | 0.461811 | 1.185223 | 0.485463 | 0.521131 | 0.871168 |
| 16 | 1.036360 | 1.201241 | 0.985163 | 0.552576 | 1.611748 | 0.792406 | 0.375426 |
| 20 | 0.193135 | 0.322870 | 0.215761 | 0.257924 | 0.273380 | 1.053934 | 0.169068 |
- The autofluorescence of E.coli KRX is not corrected.
Though we drew the graph, we couldn't find any tendency. We decide to conduct experiment with more samples and measure the autofluorescence of E.coli.
Wednesday, September 1 By: Tomonori, Tasuku
Measure OD600 of the overnight culture of C2
OD600 of the overnight culture of C2 was measured. In this experiment, C2 grew.
| Name | OD600 |
|---|---|
| C2 (diluted to 10-3) | 1.172 |
| C3 (diluted to 10-5) | 1.774 |
| C4 (diluted to 10-6) | 1.765 |
We decide not to culture of C2 for measurement of promoter activity because other bacterium can grow without antibiotics.
Restriction Digestion for to making a low copy plasmid without insert
| Name | Sample | 10xBuffer | BSA | Enzyme 1 | Enzyme 2 | MilliQ | Total | Incubation | ||
|---|---|---|---|---|---|---|---|---|---|---|
| pSB4K5 | 2.0 µL | 5.0 | 0.5 | XbaI | 0.2 | SpeI | 0.2 | 42.1 | 50.0 | At 37℃ for 2h |
- The concentration of the sample is 56ng/µL.
Ligation and Phosphorylation
After PCR purification, the sample was ligated.
| Name | Sample | Ligation High | Total |
|---|---|---|---|
| pSB4K5 | 1.0 µL | 1.0 | 2.0 |
Transformation
| Name | Sample | Competent Cell | Total | Plate | Incubation | Result |
|---|---|---|---|---|---|---|
| pSB4K5 without insert | 2.0 µL | 50.0 | 52.0 | LB (Kan+) | 09/01 - 09/02 |
Overnight culture
The ML and the MS were grown at 37℃ for a night (16 hours).
Overnight culture for the makeup test of 08/11, 08/12
| Name | Medium | IPTG |
|---|---|---|
| ML | Supplemented M9 (Kan+) | 0.00 mM |
| ML | 1.00 | |
| MS | 0.00 | |
| MS | 1.00 |
These samples were grown at 37℃ for 16 hours.
Thursday, September 2 By: Tomonori
Culture
OD600 of the overnight culture was measured.
| sample name | OD600 |
|---|---|
| ML | 1.580 |
| MS | 1.582 |
The overnight culture of ML was diluted in fresh supplemented M9 medium including 3 different concentration of IPTG and that of MS was diluted without IPTG. OD600 of each medium was measured every 4 hours. The samples to measure fluorescence of GFP were also collected.
| ML 0mM | ML 0.01mM | ML 0.1mM | MS1 | MS2 | MS3 | |
|---|---|---|---|---|---|---|
| 4h | 0.119 | 0.120 | 0.124 | 0.130 | 0.125 | 0.111 |
| 8h | 0.974 | 1.018 | 0.958 | 0.848 | 0.816 | 0.792 |
| 12h | 1.127 | 1.180 | 1.242 | 1.262 | 1.264 | 1.189 |
| 16h | 1.027 | 1.090 | 1.137 | 1.172 | 1.180 | 1.159 |
| 20h | 1.103 | 1.110 | 1.151 | 1.233 | 1.169 | 1.225 |
- Measurement at 12h, 16h and 20h was conducted the day after.
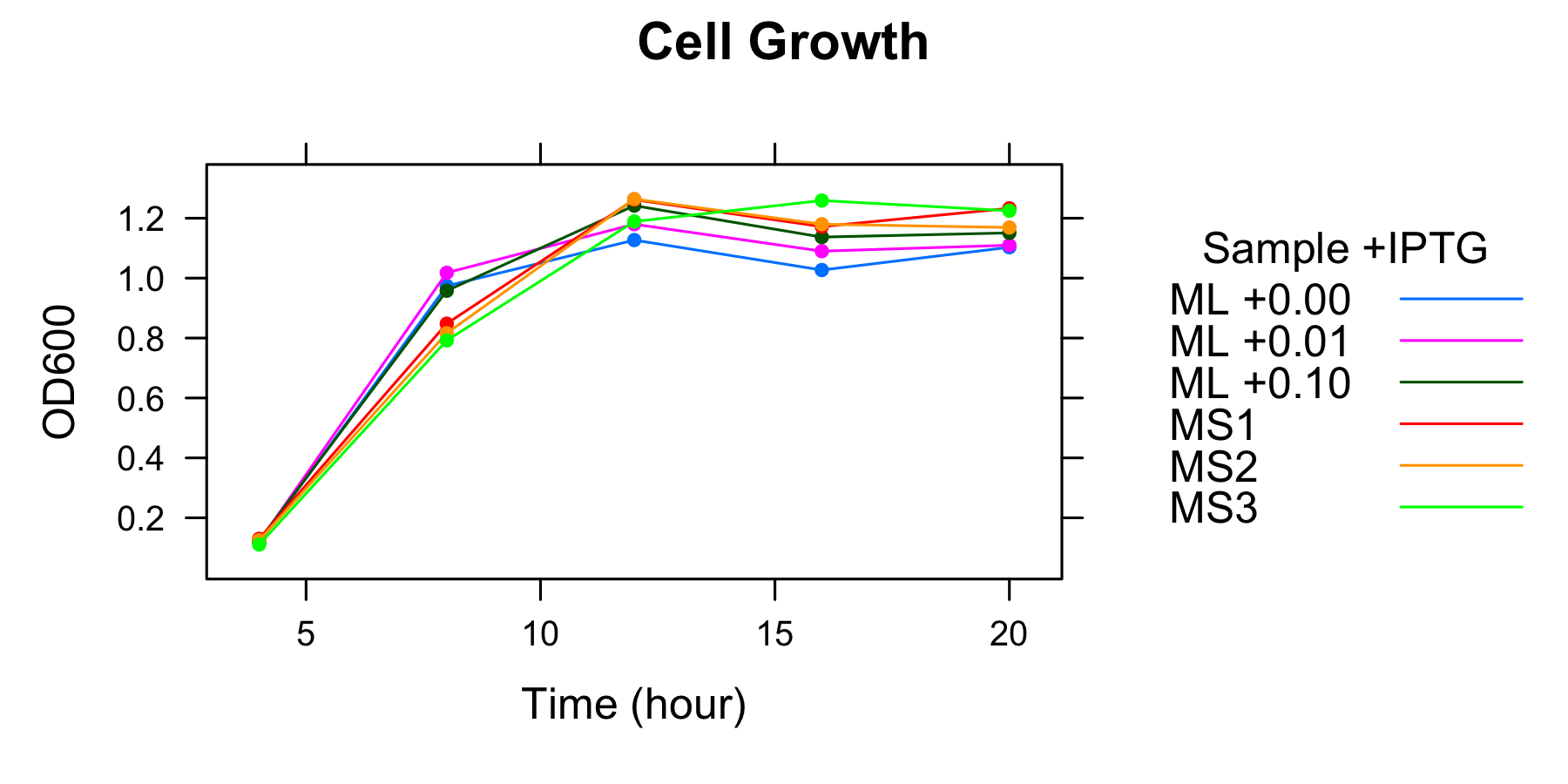 We found that the number of the cell had been in steady state at 12h and after.
We found that the number of the cell had been in steady state at 12h and after.
Culture for the makeup test of 8/11, 8/12
The overnight culture was diluted to 10-2 in the fresh culture and grown for 3hours. OD600 of the culture was measured and samples for measurement of GFP fluorescence were collected.
| sample name | OD600 |
|---|---|
| ML(IPTG 1mM) | 0.326 |
| ML(IPTG 0mM) | 0.329 |
| MS(IPTG 1mM) | 0.328 |
| MS(IPTG 0mM) | 0.314 |
Transformation of pSB4K5 without insertion
Colonies were seen on the plate.
Friday, 3 September By:Tomonori, Tasuku
Measure OD600 of the culture of MS and collect samples.
Measure the fluorescence of GFP of the ML and the MS
| ML IPTG 0mM</br> | ML IPTG 0.01mM</br> | ML IPTG 0.1mM</br> | MS1 | MS2 | MS3 | |
|---|---|---|---|---|---|---|
| 4h | 155 | 392 | 1836 | 6526 | 6572 | 8274 |
| 8h | 1046 | 1667 | 92602 | 145341 | 149358 | 119017 |
| 12h | 918 | 2733 | 119459 | 180575 | 137615 | 152878 |
| 16h | 883 | 2543 | 80473 | 138505 | 159007 | 235987 |
| 20h | 868 | 3229 | 157578 | 400829 | 152590 | 157942 |
- Back corrected
We calculate the promoter activity of the lactose promoter
| 0mM | 0.01mM | 0.1mM | |
|---|---|---|---|
| 4h | 0.022 | 0.055 | 0.251 |
| 8h | 0.006 | 0.010 | 0.575 |
| 12h | 0.006 | 0.018 | 0.758 |
| 16h | 0.006 | 0.015 | 0.465 |
| 20h | 0.004 | 0.015 | 0.703 |
- the autofluorescence of E.coli KRX is not corrected.
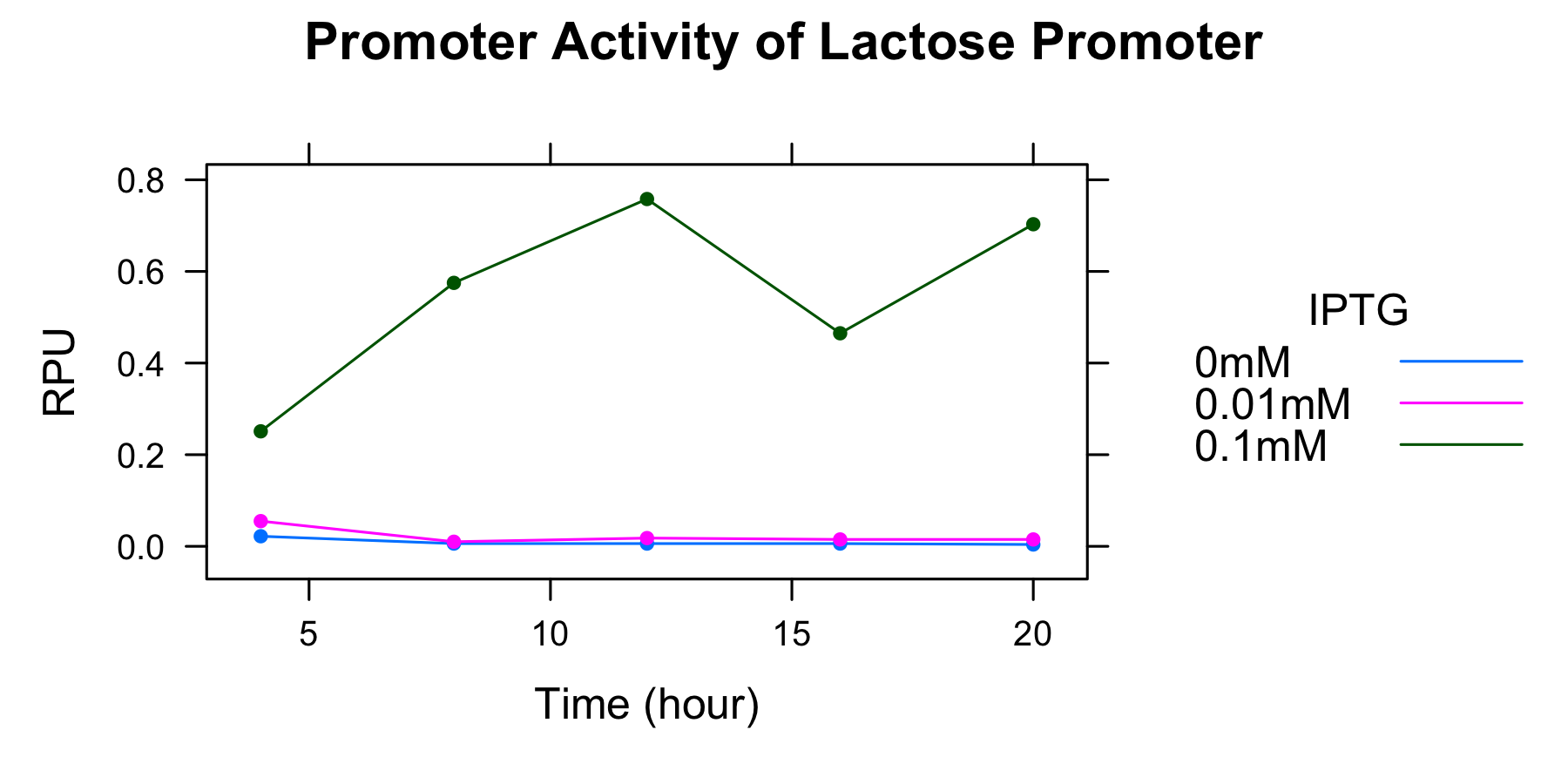 We could not find the relationship between promoter activity and incubation time.
We could not find the relationship between promoter activity and incubation time.
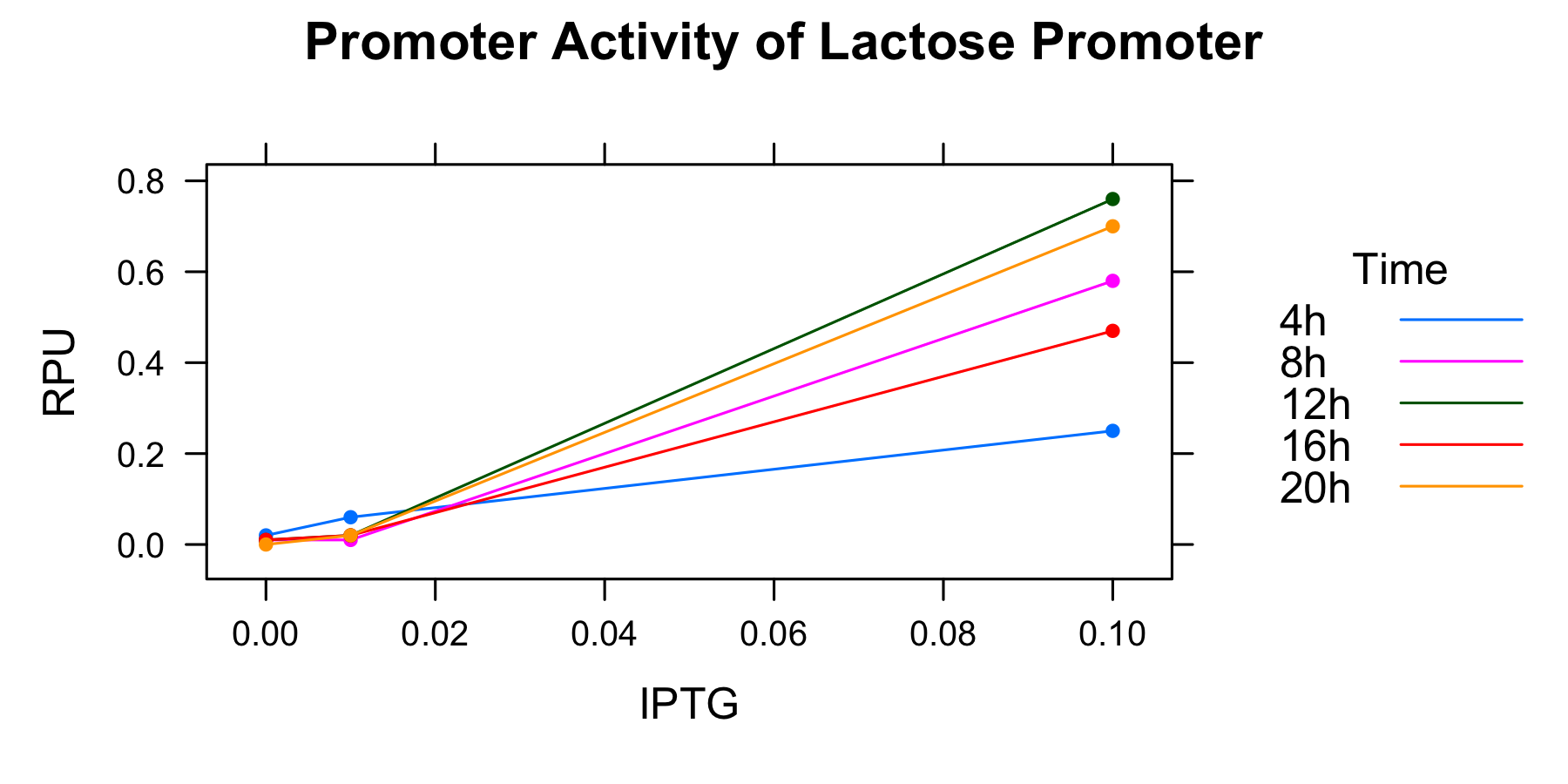 This graph indicates that the higher IPTG concentration is, the higher the activity of the lactose promoter is.
This graph indicates that the higher IPTG concentration is, the higher the activity of the lactose promoter is.
Sunday, 5 September By Kazuya
Colony PCR of pSB4K5 without insertion
Monday, 6 September By Kazuya
Electrophoresis of pSB4K5 without insertion
| Marker | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | Marker |
| lambda | pSB4K5 without insertion | 100bp | |||||||||||
The pSB4K5 must be about 2300bp. The result of the electrophoresis indicates that the transformation was failed.
Overnight culture for measurement the promoter
The ML and the MS were grown at 37℃ for a night (16 hours).
Tuesday, 7 September By: Kazuya, Tomo, Wataru, Fumitaka
Digest pSB4K5 by XbaI, SpeI to make low copy plasmid without insert again
| Sample | 10xBuffer | BSA | Enzyme1 | Enzyme2 | MilliQ | Total | Incubation |
|---|---|---|---|---|---|---|---|
| pSB4K5 2µL | 5µL | 0.5µL | XbaI 0.2µL | SpeI 0.2µL | 42.1µL | 50µL | At 37℃2h |
- the concentration of the sample, pSB4K5, is 56ng/µL
Ligation and pospholylation
After PCR purification, the sample was ligated.
| Sample | Ligation High | Total |
| pSB4K5 1µL | 1µL | 2µL |
Measure OD600 of the overnight culture
We found that the overnight culture had not grown. We think this is because we added too much antibiotics to the culture.
Wednesday, 8 September By: Tasuku, Wataru
Transformation of pSB4K5 without insert
| Sample | Conc(/µL) | Sample Volume(µL) | Competent Cell(µL) | Total | Plate | Incubation |
|---|---|---|---|---|---|---|
| pSB4K5 without insert | - | 2 | 50 | 52 | LB Kan | 9/8‾9/9 |
Overnight culture for measurement the promoter
The ML was grown at 37℃ for a night (18hours).
Thursday, 9 September By:Tasuku
Measure OD600 of the overnight culture
We measure the OD600 of overnight culture. The OD600 is 0.376.
Culture for measurement of Promoter activity
The overnight culture was diluted in fresh supplemented M9 medium including 5 different IPTG concentration and OD600 of each medium was measured every 4 hours. The samples to measure fluorescence of GFP were also collected.
| ML | 0mM | 0.01mM | 0.03mM | 0.1mM | 1mM |
|---|---|---|---|---|---|
| 4h | 0.373 | 0.325 | 0.326 | 0.359 | 0.341 |
| 8h | 1.328 | 1.355 | 1.481 | 1.405 | 1.457 |
We stopped incubation at 8h because strange floating matters were seen in the culture.
Overnight culture for measurement the promoter
The ML was grown at 37℃ for a night (16hours).
Transformation of pSB4K5 without insert
Colonies were seen on the plate.
Friday, 10 September By: Tasuku
The overnight culture was diluted in fresh supplemented M9 medium including 5 different IPTG concentration and OD600 of each medium was measured every 4 hours. The samples to measure fluorescence of GFP were also collected.
| ML | 0mM | 0.01mM | 0.03mM | 0.1mM | 1mM |
| 4h | 0.103 | 0.109 | 0.117 | 0.11 | 0.055 |
| 8h | 0.196 | 0.256 | 0.285 | 0.377 | 0.174 |
We stopped incubation at 8h because the cell growth rate was too bad.
Sunday, 12 September By: Wataru
Overnight culture of MB
MB (Measurement ? Back), that is, E.coli KRX transformed with pSB4K5 without insert was grown in the supplemented M9 medium for a night (16h).
Monday, 13 September By: Takuya Okada, Ken
Culture for measurement of the promoter activity
OD600 of MB was measured.
| 4h | 8h | 12h | 16h | 20h |
| 0.148 | 1.738 | 1.952 | 1.8 | 2.178 |
- Measurements at 12h, 16h and 20h were conducted the day after.
Tuesday, 14 September By: Kazuya, Tomonori
MeasureOD600 of MB culture and collect samples
Measure the fluorescence of the samples
| 9/9 | 9/10 | MB | |||||||||
| 0mM | 0.01mM | 0.03mM | 0.1mM | 1mM | 0mM | 0.01mM | 0.03mM | 0.1mM | 1mM | ||
| 4h | 2487 | 3322 | 4728 | 23302 | 69101 | 225 | 264 | 389 | 3391 | 5500 | 77 |
| 8h | 4472 | 11295 | 52260 | 147947 | 241965 | 1761 | 2139 | 43379 | 138454 | 187563 | 635 |
| 12h | 459 | ||||||||||
| 16h | 382 | ||||||||||
| 20h | 751 | ||||||||||
- Back corrected
We measure GFP fluorescence of the samples, but we did not these data because GFP fluorescence of ML samples might have grown abnormally.
Transformation of pSB4K5 without insert to E.coli BL21 for measurement of lactose degradation
| Sample Conc(/µL) | Sample Volume(µL) | Competent Cell(µL) | Total | Plate | Incubation | |
| pSB4K5 without insert | - | 2 | 50 | 52 | LB Kan | 9/14‾9/15 |
Overnight culture of ML for measurement promoter activity
ML in three test tubes was grown for 13hours.
Wednesday, 15 September By: Tomonori
Make the standard curve for measurement lactose concentration
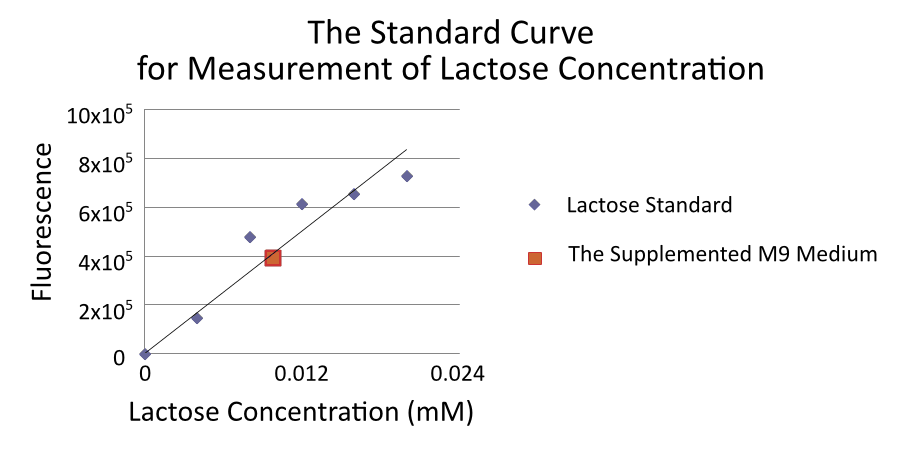 We also measure the concentration of lactose in the supplemented M9 medium and found that it contains approximately 0.01mM lactose.
We also measure the concentration of lactose in the supplemented M9 medium and found that it contains approximately 0.01mM lactose.
Thursday, 16 September By: Tomonori
Overnight culture for measurement of RPU of the lactose promoter
We changed the way of measurement of promoter activity. (to know more detail, see protocol.) Three falcon tubes of ML, three falcon tubes of MS and three falcon tubes of MB were grown with 1mM IPTG at 37℃ for 12hours.
Transformation of pSB4K5 without insert to E.coli BL21 for measurement of lactose degradation
Any colony was not seen on the plate.
Friday, 17 September By: Tomonori, Hitoshi, Takuya Okada
Measure OD600 of overnight culture
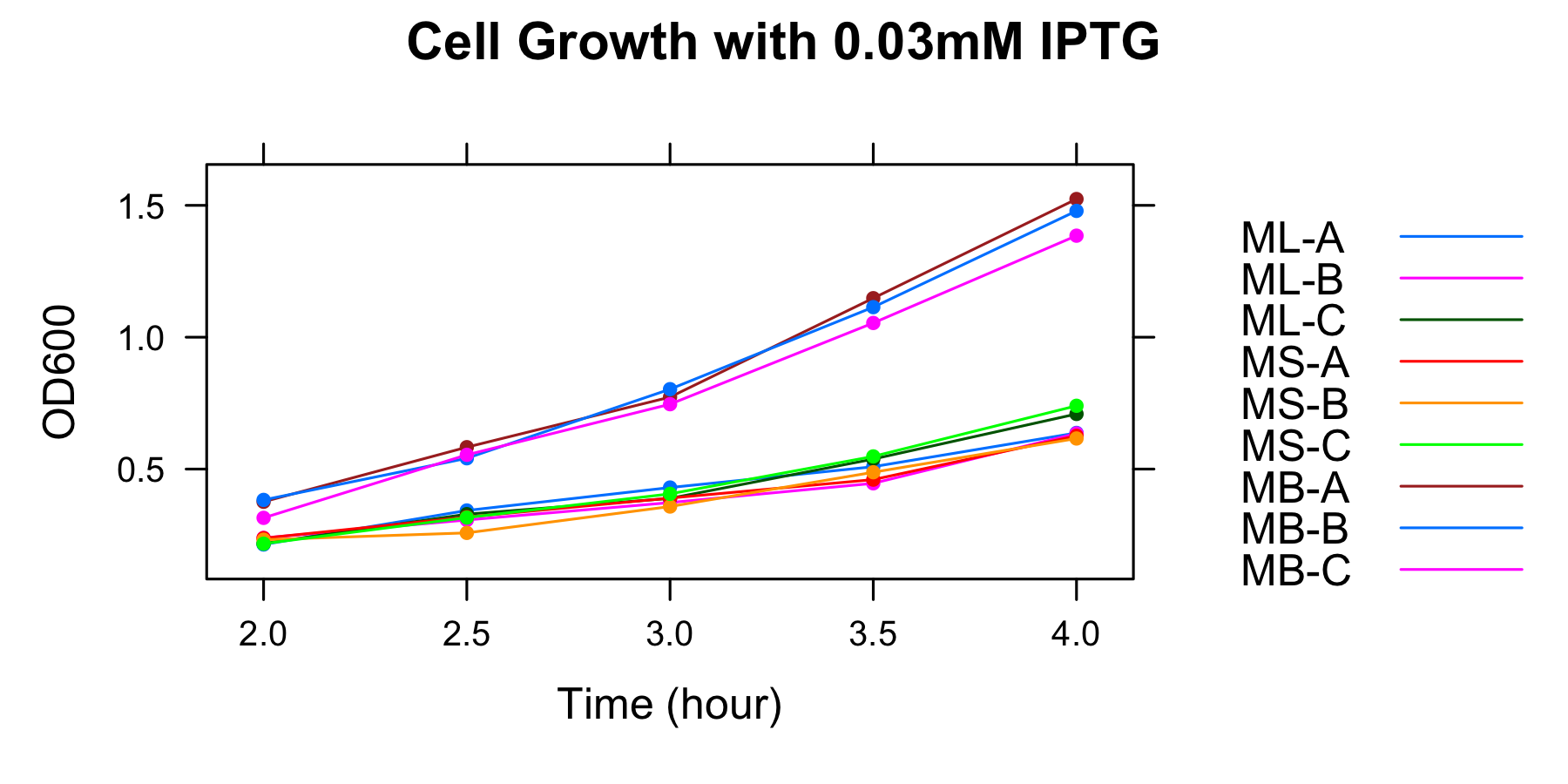
| sample name | ML-A | ML-B | ML-C | MS-A | MS-B | MS-C | MB-A | MB-B | MB-C |
| OD600 | 1.900 | 1.745 | 1.937 | 1.905 | 1.938 | 1.488 | 3.276 | 3.300 | 3.202 |
Culture for measurement of RPU of the lactose promoter
Overnight culture was diluted in the fresh medium including 1mM IPTG and OD600 was measured at 1h, 2h, 3h, 3.5h, 4h.
| OD600 | ML-A | ML-B | ML-C | MS-A | MS-B | MS-C | MB-A | MB-B | MB-C |
|---|---|---|---|---|---|---|---|---|---|
| 1h | 0.017 | 0.103 | 0.118 | 0.057 | 0.073 | 0.044 | -0.028 | 0.101 | 0.098 |
| 2h | 0.270 | 0.295 | 0.245 | 0.167 | 0.241 | 0.149 | 0.208 | 0.179 | 0.294 |
| 3h | 0.33 | 0.33 | 0.35 | 0.36 | 0.42 | 0.31 | 0.66 | 0.70 | 0.66 |
| 3.5h | 0.47 | 0.37 | 0.46 | 0.49 | 0.55 | 0.40 | 0.98 | 0.98 | 0.96 |
| 4h | 0.675 | 0.595 | 0.665 | 0.684 | 0.793 | 0.607 | 1.358 | 1.362 | 1.360 |
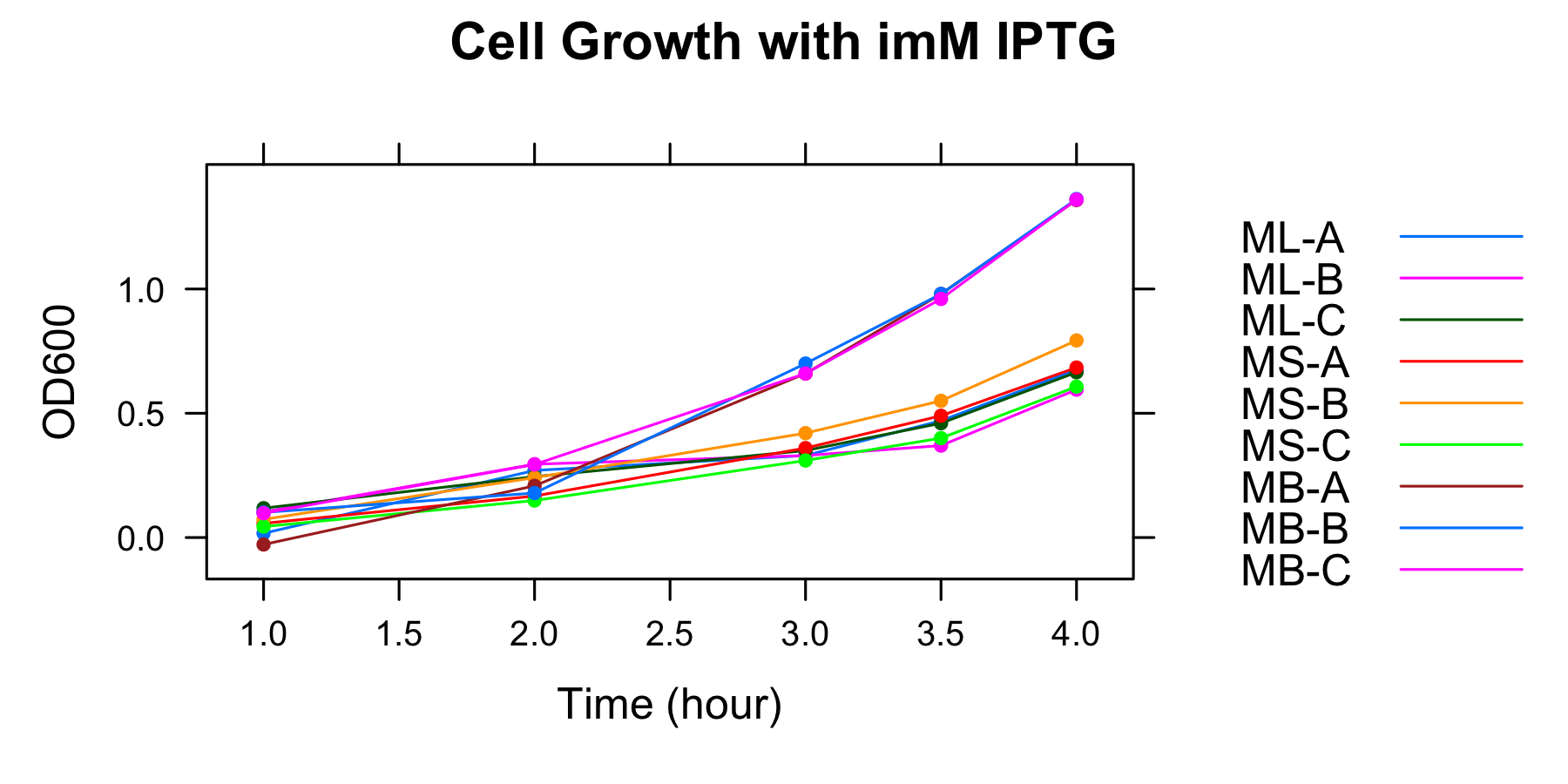 This graph indicates that all samples were in the exponential growth from 2h to 4h.
The samples for measurement of GFP fluorescence were collected at 3h and 3.5h.
This graph indicates that all samples were in the exponential growth from 2h to 4h.
The samples for measurement of GFP fluorescence were collected at 3h and 3.5h.
| GFP | ML-A | ML-B | ML-C | MS-A | MS-B | MS-C | MB-A | MB-B | MB-C |
| 3h | 58603 | 49991 | 50845 | 53416 | 55463 | 40321 | 204 | 167 | 152 |
| 3.5h | 89428 | 76573 | 85433 | 67457 | 83329 | 59463 | 218 | 271 | 246 |
Back corrected We calculate the RPU of the lactose promoter with 1mM IPTG is 1.65.
| ML-A | ML-B | ML-C | average | stdev | CV | |
| RPU | 1.60 | 1.58 | 1.77 | 1.65 | 0.106 | 0.0642 |
Overnight culture of the ML was also diluted in the fresh medium including 0mM IPTG, 0.01mM IPTG, 0.03mM IPTG, 0.1mM IPTG and 1mM IPTG and the samples for measurement of GFP fluorescence were collected at 3h and 3.5h.
| IPTG 0mM | ML-A | ML-B | ML-C |
| 3h | 1906 | 2138 | 2190 |
| 3.5h | 1781 | 1141 | 980 |
| IPTG 0.01mM | ML-A | ML-B | ML-C |
| 3h | 5037 | 2588 | 2189 |
| 3.5h | 1191 | 1700 | 1511 |
| IPTG 0.03mM | ML-A | ML-B | ML-C |
| 3h | 2525 | 2410 | 3065 |
| 3.5h | 1982 | 3330 | 3723 |
| IPTG 0.1mM | ML-A | ML-B | ML-C |
| 3h | 14191 | 9154 | 9856 |
| 3.5h | 6897 | 5427 | 7084 |
- Back corrected
We did not calculate RPU from these data because GFP fluorescence at 3.5h was lower than that at 3h in most of the samples, and we couldn't calculate GFP synthesis rate. We think this is because GFP synthesized in overnight culture with IPTG 1mM had stayed more at 3h than 3.5h.
Make a new plate of the MB
Monday, 20 September By: Tomonori
Make the supplemented M9 medium without casamino acids
We think that lactose in the supplemented M9 medium come from casamino acids, so we decide to measure RPU of the lactose promoter without casamino acids because lactose in medium induces the lactose promoter.
Overnight culture for measurement of RPU of the lactose promoter with IPTG 0.1mM
Three falcon tubes of the ML, three falcon tubes of the MS and three falcon tubes of the MB were grown in the supplemented M9 medium without casamino acids including 0.1mM IPTG at 37℃ for 23 hours.
Tuesday, 21 September By: Hitoshi
Measure OD600 of overnight culture
| sample name | ML-A | ML-B | ML-C | MS-A | MS-B | MS-C | MB-A | MB-B | MB-C |
| OD600 | 2.140 | 2.080 | 2.103 | 1.975 | 1.964 | 1.993 | 0.041 | 0.062 | 0.025 |
We found that MB had not grown.
Culture for measurement of RPU of the lactose promoter
ML and MS were cultured and OD600 was measured at 2h, 2.5h, 3h, 3.5h and 4h in the medium without casamino acids. The samples were collected at 3h and 3,5h.
| OD600 IPTG 0.1mM</br> | ML-A | ML-B | ML-C | MS-A | MS-B | MS-C |
|---|---|---|---|---|---|---|
| 2h | -0.056 | -0.002 | 0.004 | 0.027 | -0.001 | 0.024 |
| 2.5h | -0.023 | 0.000 | 0.014 | -0.050 | 0.003 | 0.0025 |
| 3h | -0.050 | 0.003 | 0.0025 | 0.0036 | 0.009 | 0.040 |
| 3.5h | -0.036 | 0.002 | 0.041 | 0.061 | 0.061 | 0.039 |
| 4h | -0.030 | 0.045 | 0.04 | 0.0175 | 0.074 | 0.104 |
We found that ML and MS had grown hardly.
Overnight culture for measurement of RPU of the lactose promoter with IPTG 0mM
Three falcon tubes of ML, three falcon tubes of MS and three falcon tubes of MB were grown in the supplemented M9 medium without casamino acids including 0mM IPTG and 0.01mM ITPG at 37℃ for 23 hours.
Wednesday, 22 September By: Hitoshi, Takuya Okada
Measure OD600 of overnight culture
| IPTG 0mM | ML-A | ML-B | ML-C | MS-A | MS-B | MS-C | MB-A | MB-B | MB-C |
| OD600 | 2.009 | 1.948 | 1.965 | 2.168 | 1.951 | 1.719 | 0.042 | 0.052 | 0.032 |
| IPTG 0.01mM | ML-A | ML-B | ML-C | MS-A | MS-B | MS-C | MB-A | MB-B | MB-C |
| OD600 | 2.091 | 1.414 | 2.082 | 1.460 | 1.959 | 2.099 | 0.035 | 0.050 | 0.042 |
We found that MB had not grown.
Culture for measurement of RPU of the lactose promoter
ML and MS were cultured and OD600 was measured at 2h, 2.5h, 3h, 3.5h and 4h in the medium without casamino acids. The samples were collected at 3h and 3,5h.
| OD600 IPTG 0mM</br> | ML-A | ML-B | ML-C | MS-A | MS-B | MS-C |
| 2h | 0.099 | 0.102 | 0.119 | 0.080 | 0.082 | 0.068 |
| 2.5h | 0.115 | 0.141 | 0.136 | 0.092 | 0.068 | 0.110 |
| 3h | 0.176 | 0.173 | 0.193 | 0.130 | 0.093 | 0.124 |
| 3.5h | 0.236 | 0.237 | 0.248 | 0.201 | 0.129 | 0.169 |
| 4h | 0.320 | 0.249 | 0.256 | 0.243 | 0.156 | 0.232 |
| OD600 IPTG 0.01mM</br> | ML-A | ML-B | ML-C | MS-A | MS-B | MS-C |
| 2h | 0.135 | 0.070 | 0.116 | 0.060 | 0.075 | 0.092 |
| 2.5h | 0.155 | 0.091 | 0.142 | 0.071 | 0.103 | 0.127 |
| 3h | 0.167 | 0.092 | 0.196 | 0.080 | 0.148 | 0.168 |
| 3.5h | 0.227 | 0.180 | 0.331 | 0.151 | 0.230 | 0.271 |
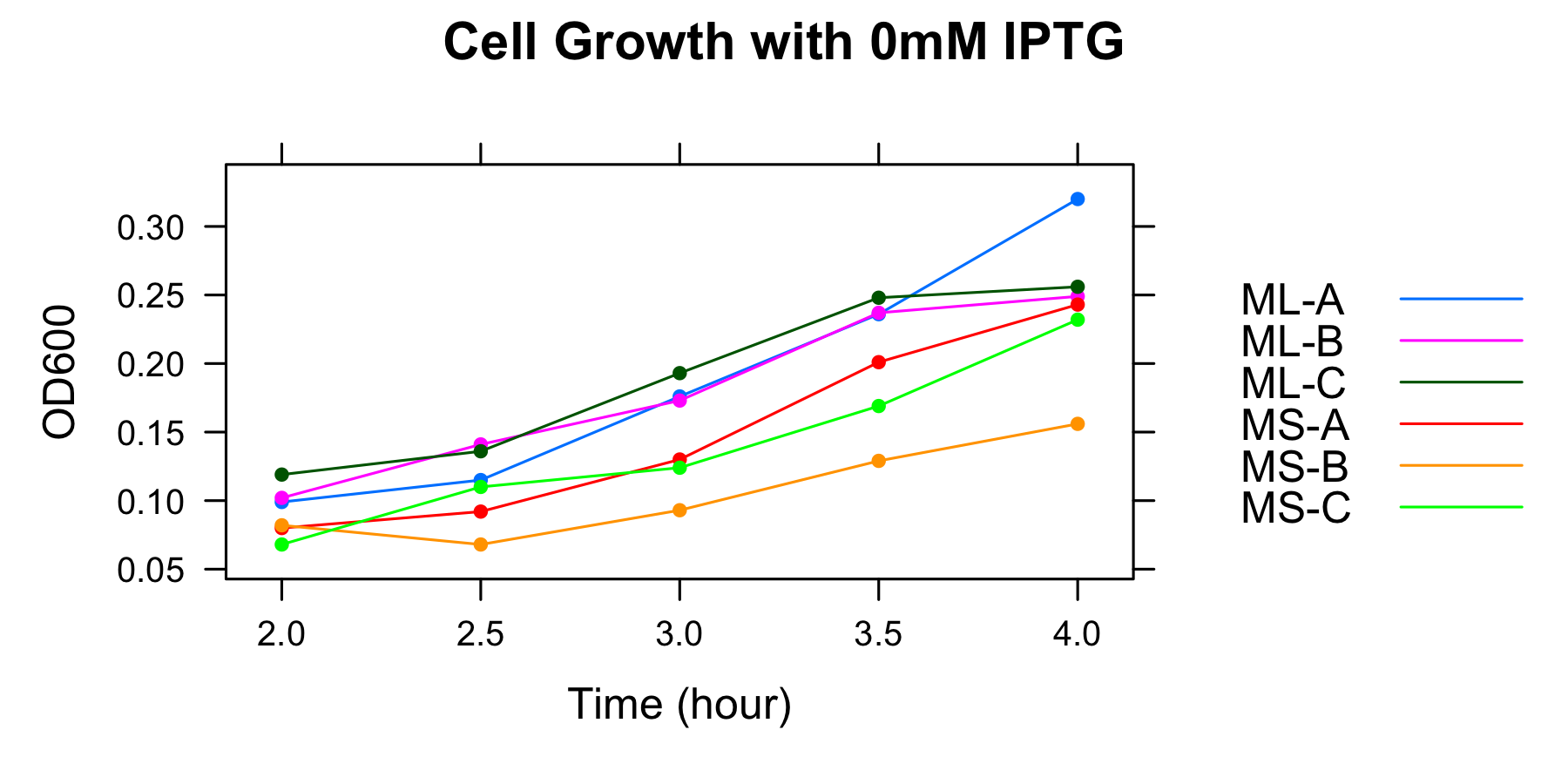
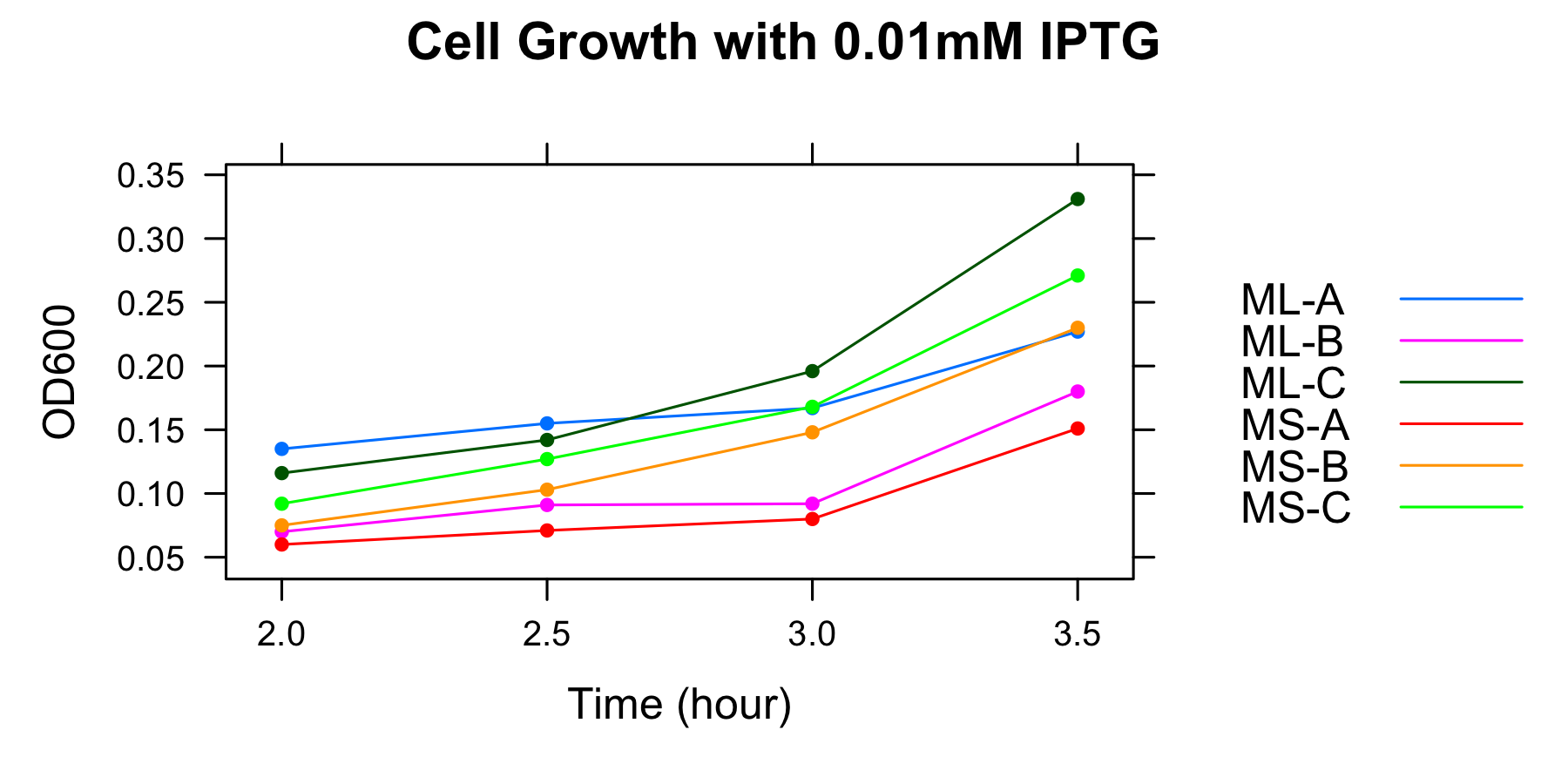 We found that cell growth rate was low without casamino acids and that OD600 of the culture at 3h was low, therefore we decide to measure OD600 at 4h, 4.5h, 5h, 5.5h and 6h, and to collect samples at 5h and 5.5h in later experiment without casamino acids.
We found that cell growth rate was low without casamino acids and that OD600 of the culture at 3h was low, therefore we decide to measure OD600 at 4h, 4.5h, 5h, 5.5h and 6h, and to collect samples at 5h and 5.5h in later experiment without casamino acids.
Overnight culture for the measurement of lactose promoter
We cultivated ML and MS in the supplemented M9 medium without casamino acids including 0.03mM IPTG and 0.1mM IPTG.
Thursday, 23 September By: Hitoshi, Kazuya
Measure OD600 of the overnight culture
| 0.03mM | ML | MS |
| OD600 | 1.417 | 1.483 |
| 0.1mM | ML | MS |
| OD600 | 2.126 | 2.131 |
Culture for measurement of RPU of the lactose promoter
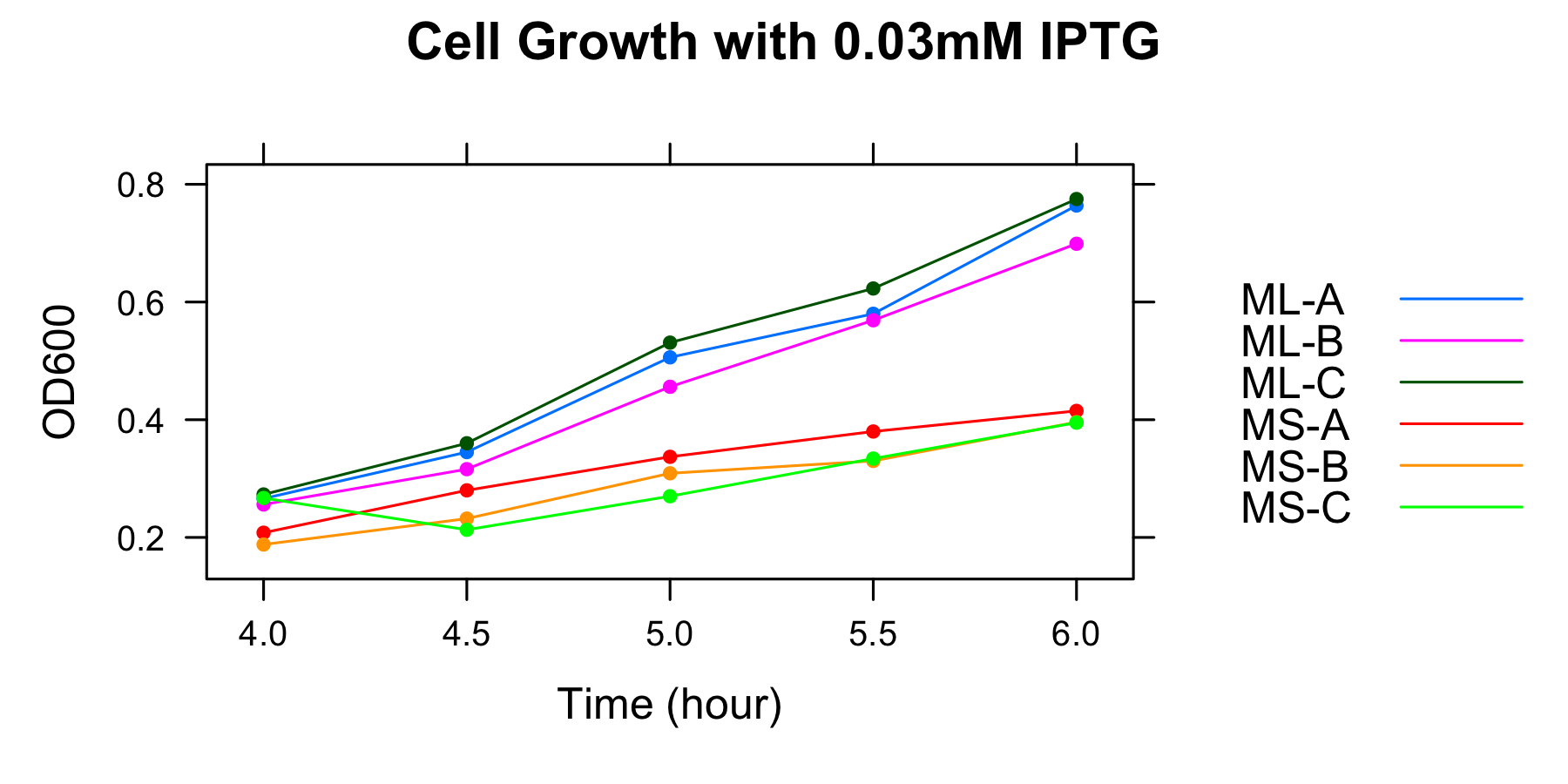
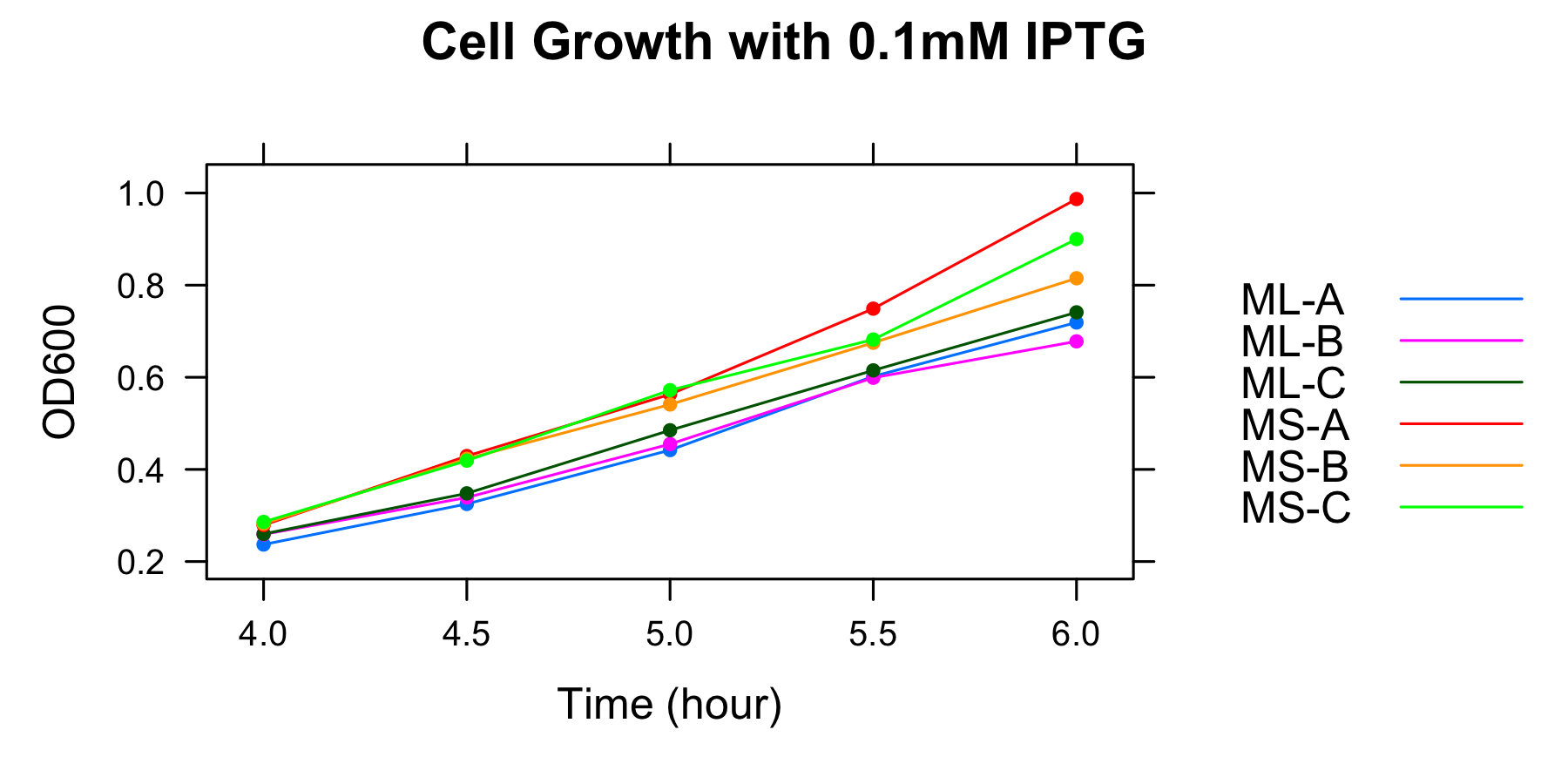
ML and MS were cultured and OD600 was measured at 4h, 4.5h, 5h, 5.5h and 6h in the medium without casamino acids. The samples were collected at 5h and 5.5h.
| 0.03mM | ML-A | ML-B | ML-C | MS-A | MS-B | MS-C |
| 4h | 0.266 | 0.256 | 0.273 | 0.208 | 0.188 | 0.267 |
| 4.5h | 0.345 | 0.316 | 0.360 | 0.280 | 0.232 | 0.213 |
| 5h | 0.506 | 0.456 | 0.531 | 0.337 | 0.309 | 0.270 |
| 5.5h | 0.580 | 0.569 | 0.623 | 0.380 | 0.330 | 0.334 |
| 6h | 0.764 | 0.699 | 0.775 | 0.415 | 0.396 | 0.395 |
| 0.1mM | ML-A | ML-B | ML-C | MS-A | MS-B | MS-C |
| 4h | 0.237 | 0.259 | 0.26 | 0.279 | 0.282 | 0.286 |
| 4.5h | 0.325 | 0.339 | 0.348 | 0.429 | 0.423 | 0.419 |
| 5h | 0.442 | 0.455 | 0.485 | 0.563 | 0.541 | 0.572 |
| 5.5h | 0.602 | 0.599 | 0.615 | 0.749 | 0.675 | 0.682 |
| 6h | 0.719 | 0.678 | 0.741 | 0.987 | 0.815 | 0.900 |
Overnight culture for the measurement of lactose promoter
We cultivated ML and MS in the supplemented M9 medium without casamino acids including 1mM IPTG.
Friday, 24 September By: Tomonori, Hitoshi, Tasuku, Takuya Okada, Ken, Kazuya
Measure OD600 of the overnight culture
| 1mM | ML | MS |
| OD600 | 1.187 | 1.427 |
Culture for measurement of RPU of the lactose promoter
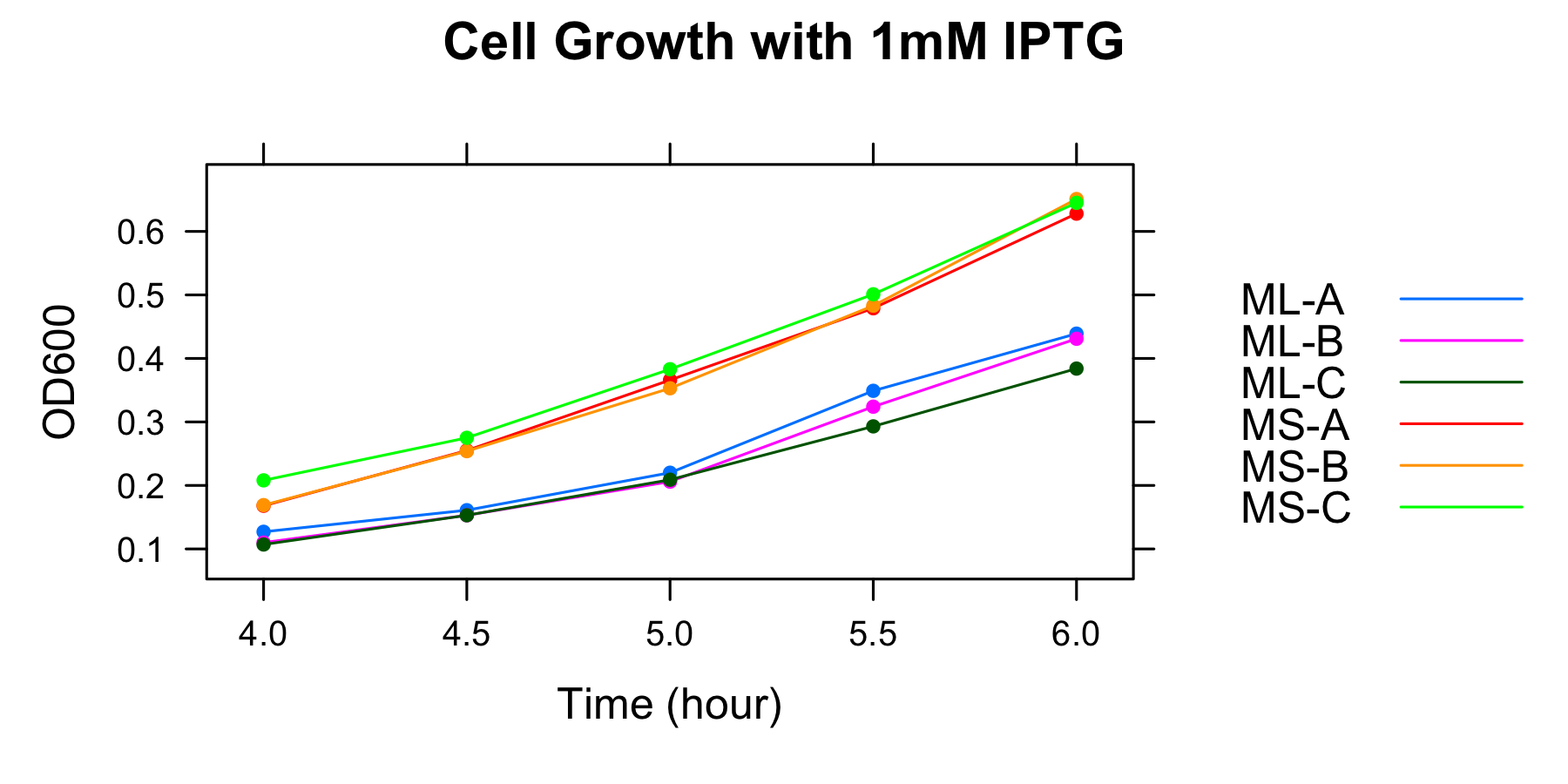
ML and MS were cultured and OD600 was measured at 4h, 4.5h, 5h, 5.5h and 6h in the medium without casamino acids. The samples were collected at 5h and 5.5h.
| 1mM | ML-A | ML-B | ML-C | MS-A | MS-B | MS-C |
| 4h | 0.127 | 0.11 | 0.107 | 0.168 | 0.169 | 0.208 |
| 4.5h | 0.161 | 0.153 | 0.153 | 0.255 | 0.254 | 0.275 |
| 5h | 0.220 | 0.206 | 0.209 | 0.366 | 0.353 | 0.383 |
| 5.5h | 0.349 | 0.324 | 0.293 | 0.479 | 0.483 | 0.501 |
| 6h | 0.439 | 0.431 | 0.384 | 0.628 | 0.651 | 0.645 |
Measure the fluorescence of GFP of the ML and the MS
| 0mM | ML-A | ML-B | ML-C | MS-A | MS-B | MS-C |
| 3h | 292 | 263 | 88 | 6086 | 3007 | 6443 |
| 3.5h | 131 | 93 | -45 | 1700 | 1676 | 1652 |
| 0.01mM | ML-A | ML-B | ML-C | MS-A | MS-B | MS-C |
| 3h | 124 | 42 | 378 | 3425 | 6640 | 5990 |
| 3.5h | 330 | -41 | 632 | 5830 | 10515 | 13601 |
| 0.03mM | ML-A | ML-B | ML-C | MS-A | MS-B | MS-C |
| 5h | 48848 | 23900 | 51150 | 38837 | 13416 | 33454 |
| 5.5h | 54980 | 54980 | 54980 | 35758 | 34595 | 34443 |
| 0.1mM 20 September</br> | ML-A | ML-B | ML-C | MS-A | MS-B | MS-C |
| 3h | 31 | 3327 | 2219 | 3331 | 2298 | 3766 |
| 3.5h | 61 | 5341 | 3489 | 4840 | 3955 | 4512 |
| 0.1mM 23 September</br> | ML-A | ML-B | ML-C | MS-A | MS-B | MS-C |
| 5h | 72902 | 68382 | 78054 | 56365 | 42563 | 57917 |
| 5.5h | 98103 | 84737 | 81525 | 89239 | 91295 | 68302 |
Back corrected We can't use the data of IPTG 0mM to calculate RPU because GFP fluorescence at 3.5h is lower than that at 3h. Also we can't use the data of ML-B 0.01mM IPTG and the data of MS-A of 0.03mM. Although GFP fluorescence of 0.1mM (20 September) at 3.5h is higher than that at 3h, OD600 is so low. Accordingly, We can't use the data of 0.1mM (20 September).
Overnight culture for the measurement of lactose promoter
Three tubes of the ML, three tubes of the MS and three tubes of the MB were grown with casamino acids including 0.1mM ITPG and 0.01mM IPTG for 16hours.
Saturday, 25 September By: Hitoshi, Tomonori
Measure OD600 of the overnight culture
| 0.1mM | ML-A | ML-B | ML-C | MS-A | MS-B | MS-C | MB-A | MB-B | MB-C |
| OD600 | 1.961 | 1.823 | 2.078 | 2.241 | 2.383 | 1.857 | 2.674 | 2.674 | 2.632 |
| 0.01mM | ML-A | ML-B | ML-C | MS-A | MS-B | MS-C | MB-A | MB-B | MB-C |
| OD600 | 1.754 | 1.868 | 1.702 | 1.885 | 2.009 | 1.940 | 2.693 | 2.631 | 2.622 |
Culture for measurement of RPU of the lactose promoter
Overnight culture was diluted in the fresh medium and OD600 was measured at 2h, 2.5h, 3h, 3.5h and 4h. The samples for measurement of GFP fluorescence were collected at 3h and 3.5h.
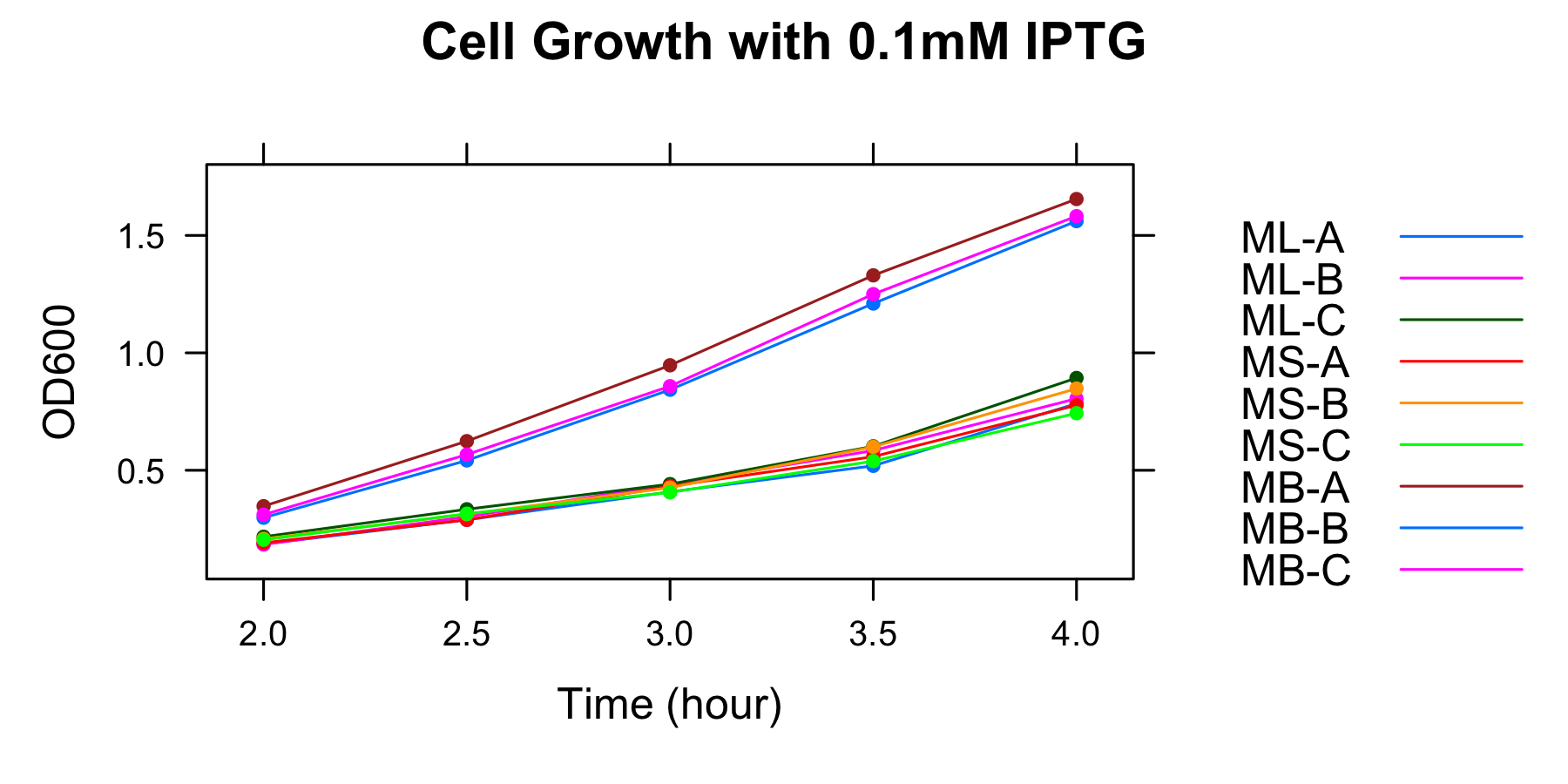
| 0.1mM | ML-A | ML-B | ML-C | MS-A | MS-B | MS-C | MB-A | MB-B | MB-C |
| 2h | 0.186 | 0.184 | 0.217 | 0.191 | 0.208 | 0.204 | 0.347 | 0.298 | 0.311 |
| 2.5h | 0.289 | 0.302 | 0.334 | 0.289 | 0.313 | 0.315 | 0.624 | 0.542 | 0.567 |
| 3h | 0.408 | 0.44 | 0.441 | 0.432 | 0.426 | 0.406 | 0.947 | 0.843 | 0.858 |
| 3.5h | 0.519 | 0.584 | 0.602 | 0.558 | 0.6 | 0.539 | 1.33 | 1.21 | 1.25 |
| 4h | 0.784 | 0.805 | 0.893 | 0.777 | 0.848 | 0.743 | 1.655 | 1.561 | 1.582 |
Overnight culture for the measurement of lactose promoter
Three tubes of the ML, three tubes of the MS and three tubes of the MB were grown with casamino acids including 0mM ITPG.
Sunday, 26 September By: Tomonori
Measure OD600 of the overnight culture
| 0mM | ML-A | ML-B | ML-C | MS-A | MS-B | MS-C | MB-A | MB-B | MB-C |
| OD600 | 1.646 | 1.576 | 1.643 | 1.702 | 1.832 | 1.826 | 2.272 | 2.090 | 1.985 |
Culture for measurement of RPU of the lactose promoter
Overnight culture was diluted in the fresh medium and OD600 was measured at 2h, 2.5h, 3h, 3.5h and 4h. The samples for measurement of GFP fluorescence were collected at 3h and 3.5h.
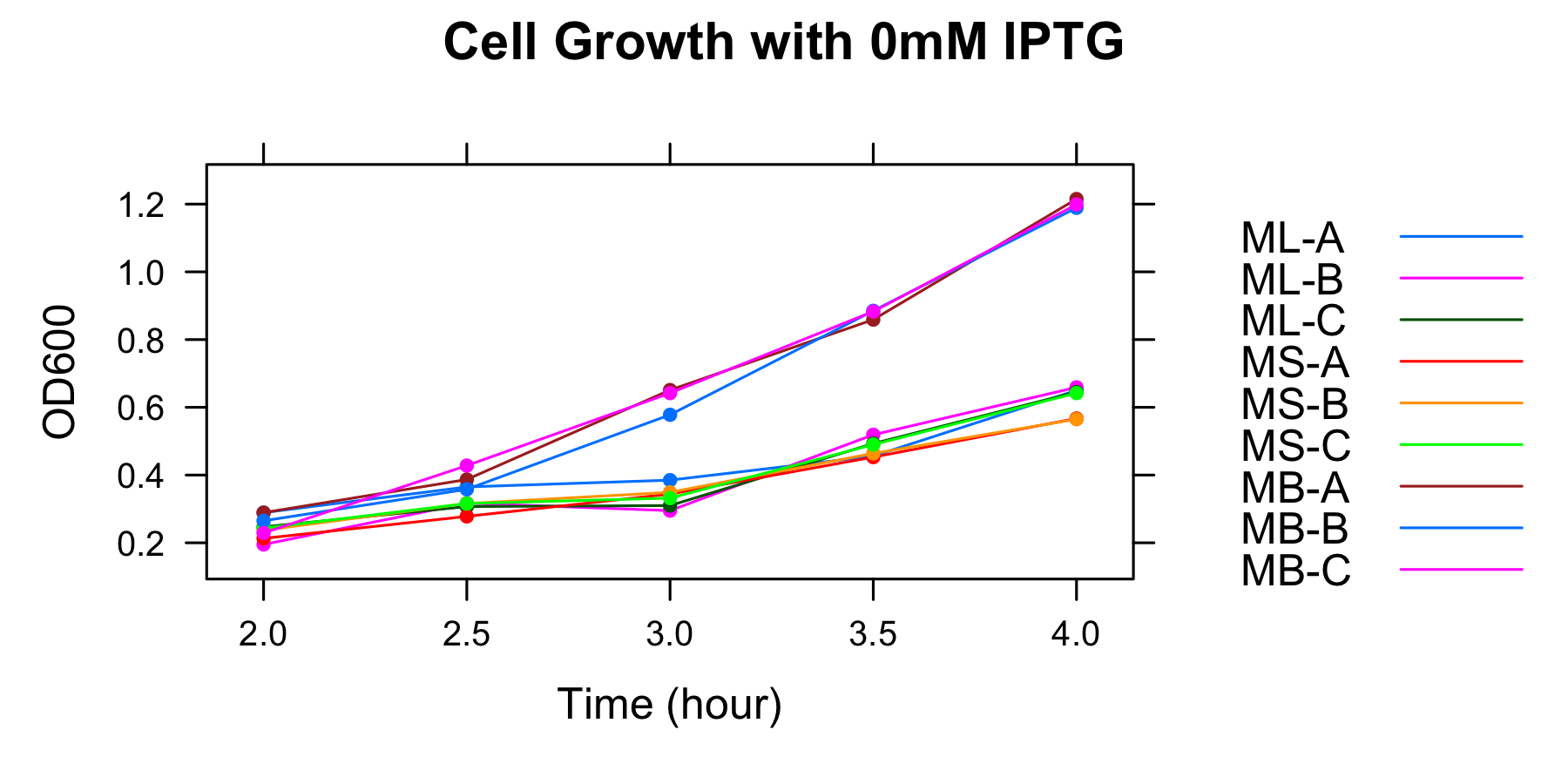
| 0mM | ML-A | ML-B | ML-C | MS-A | MS-B | MS-C | MB-A | MB-B | MB-C |
| 2h | 0.289 | 0.195 | 0.247 | 0.213 | 0.237 | 0.243 | 0.289 | 0.265 | 0.229 |
| 2.5h | 0.365 | 0.317 | 0.307 | 0.278 | 0.316 | 0.316 | 0.387 | 0.358 | 0.428 |
| 3h | 0.385 | 0.295 | 0.31 | 0.344 | 0.349 | 0.332 | 0.651 | 0.578 | 0.642 |
| 3.5h | 0.456 | 0.519 | 0.493 | 0.453 | 0.464 | 0.49 | 0.859 | 0.885 | 0.883 |
| 4h | 0.65 | 0.659 | 0.645 | 0.567 | 0.565 | 0.642 | 1.215 | 1.189 | 1.199 |
Overnight culture for the measurement of lactose promoter
Three tubes of the ML, three tubes of the MS and three tubes of the MB were grown with casamino acids including 0.03mM ITPG.
Monday, 27 September By: Tomonori, Tasuku, Ken
Measure OD600 of the overnight culture
| 0.03mM | ML-A | ML-B | ML-C | MS-A | MS-B | MS-C | MB-A | MB-B | MB-C |
| OD600 | 1.646 | 1.576 | 1.643 | 1.702 | 1.832 | 1.826 | 2.272 | 2.090 | 1.985 |
Culture for measurement of RPU of the lactose promoter
Overnight culture was diluted in the fresh medium and OD600 was measured at 2h, 2.5h, 3h, 3.5h and 4h. The samples for measurement of GFP fluorescence were collected at 3h and 3.5h.
| 0.03mM | ML-A | ML-B | ML-C | MS-A | MS-B | MS-C | MB-A | MB-B | MB-C |
| 2h | 0.214 | 0.239 | 0.218 | 0.238 | 0.232 | 0.217 | 0.376 | 0.383 | 0.315 |
| 2.5h | 0.343 | 0.307 | 0.329 | 0.318 | 0.258 | 0.316 | 0.583 | 0.541 | 0.554 |
| 3h | 0.43 | 0.373 | 0.389 | 0.39 | 0.358 | 0.406 | 0.773 | 0.803 | 0.746 |
| 3.5h | 0.509 | 0.446 | 0.538 | 0.46 | 0.488 | 0.548 | 1.148 | 1.114 | 1.054 |
| 4h | 0.637 | 0.635 | 0.709 | 0.626 | 0.616 | 0.74 | 1.524 | 1.479 | 1.385 |
Measure the GFP fluorescence
| 0mM | ML-A | ML-B | ML-C | MS-A | MS-B | MS-C | MB-A | MB-B | MB-C |
| 3h | 589 | 508 | 417 | 43120 | 40563 | 50343 | -368 | 230 | 255 |
| 3.5h | 704 | 938 | 868 | 58066 | 66566 | 65910 | 346 | 765 | 508 |
| 0.01mM | ML-A | ML-B | ML-C | MS-A | MS-B | MS-C | MB-A | MB-B | MB-C |
| 3h | 1187 | 1316 | 1329 | 59806 | 69376 | 62702 | 360 | 396 | 439 |
| 3.5h | 1883 | 1707 | 1507 | 96634 | 93095 | 88262 | 583 | 559 | 588 |
| 0.03mM | ML-A | ML-B | ML-C | MS-A | MS-B | MS-C | MB-A | MB-B | MB-C |
| 3h | 2341 | 884 | 2575 | 34995 | 33056 | 14529 | 273 | 171 | 268 |
| 3.5h | 3219 | 3124 | 1243 | 18855 | 20536 | 14040 | 416 | 230 | 209 |
| 0.1mM | ML-A | ML-B | ML-C | MS-A | MS-B | MS-C | MB-A | MB-B | MB-C |
| 3h | 17202 | 20387 | 25090 | 61144 | 13667 | 54117 | 313 | 401 | 388 |
| 3.5h | 22040 | 25372 | 32237 | 87691 | 89368 | 76786 | 613 | 338 | 476 |
Back corrected We did not use the data in grey cells of the above table.
| RPU | ML-A | ML-B | ML-C | average | stdev | CV |
| 0mM | -0.00062 | 0.0122 | 0.0147 | 0.0134 | 0.00821 | 0.611 |
| 0.01mM | 0.0191 | 0.00963 | 0.00244 | 0.0144 | 0.00838 | 0.583 |
| 0.1mM | 0.205 | 0.191 | 0.269 | 0.221 | 0.0415 | 0.188 |
We did not use the data in grey cells of the above table. CV of RPU of 0mM IPTG and that of 0.01mM IPTG is high. In addition, the supplemented M9 medium used in the experiment includes 0.01mM lactose. The RPU of the lactose promoter induced by IPTG only must be lower than the data resulted from this experiment.
 "
"