Team:Michigan/Protocols
From 2010.igem.org
Revision as of 17:51, 17 October 2010 by Chillywings (Talk | contribs)
Protocols
Using Lab Equipment
Obtaining Deionized Water in the ERB
Enigeering Research Building Autoclave
Epifluorescence Microscope Usage - H.H. Dow
Cell Culture
Making cultures from a -80C freezer stock
P. putida KT2440 antibotic resistance tolerance
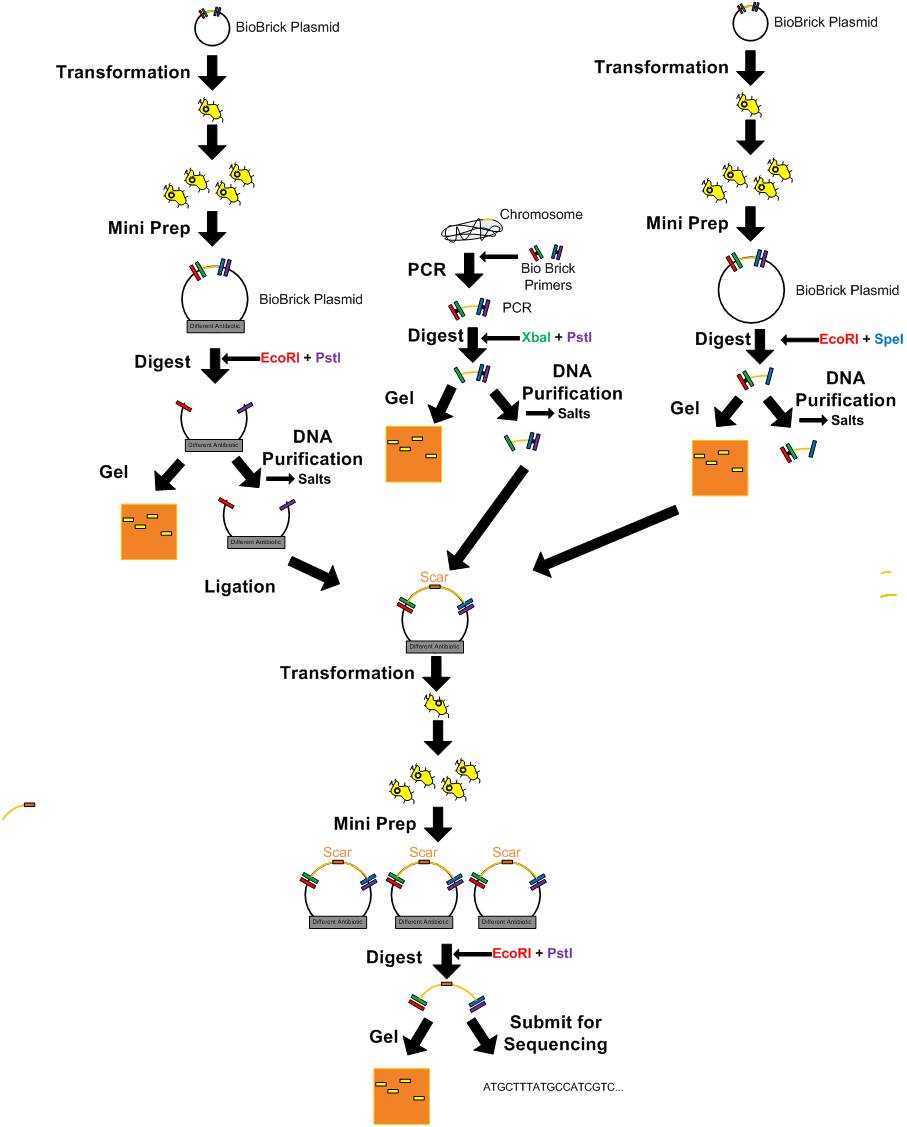
DNA Manipulation
Direct Plating Transformation Protocol
- Very simple protocol
- Twice as efficient as standard heat shock
- Getting parts from the 360 well registry plates
- Competent cell preparation
- Heat shock
Transformation-electroporation
- Getting parts from the 360 well registry plates
- Competent cell preparation
- Electroporation
- Adopted from Jeremy Minty's protocol
- General protocol with specific instructions for ERB/Lin lab equipment
Updated T4 DNA Ligase Protocol (Not quick ligase)
- For additional information/questions, refer to [http://www.neb.com/nebecomm/tech_reference/modifying_enzymes/ligation_tips.asp NEB Ligation Tips]
- Ligation
- Ginko Bioworks Protocol
- Jeremy Minty Protocol
Group-Specific Protocols
Oil Sands
Revised biofilm assay protocol
- Updated Colony PCR Protocol
 "
"