Team:Michigan/Oil Sands
From 2010.igem.org
Chillywings (Talk | contribs) (→10/7/2010) |
Chillywings (Talk | contribs) |
||
| Line 1,367: | Line 1,367: | ||
Surprisingly, they seem to grow better at pH 9, at least in co-culture. As expected, the co-culture did better than pure cultures. This lends some support towards Alex's biofilm assay results, however, it should still be repeated. Also, plated a serial dilution of the pH 9 co-culture, at 10<sup>-5</sup>, 10<sup>-6</sup>, and 10<sup>-7</sup> dilutions to determine the conversion factor between OD600 and cell count. Made streak plates of all other cultures, pure cultures for stock, and pH 7 co-culture to determine if colonies could be distinguished. All cultures and plates were returned to the 30<sup>o</sup>C incubator, without shaking. | Surprisingly, they seem to grow better at pH 9, at least in co-culture. As expected, the co-culture did better than pure cultures. This lends some support towards Alex's biofilm assay results, however, it should still be repeated. Also, plated a serial dilution of the pH 9 co-culture, at 10<sup>-5</sup>, 10<sup>-6</sup>, and 10<sup>-7</sup> dilutions to determine the conversion factor between OD600 and cell count. Made streak plates of all other cultures, pure cultures for stock, and pH 7 co-culture to determine if colonies could be distinguished. All cultures and plates were returned to the 30<sup>o</sup>C incubator, without shaking. | ||
| + | |||
| + | == 10/8/2010 == | ||
| + | ''Marcus'' | ||
| + | |||
| + | Kevin removed the transformation plates which were placed back in the incubator to the 4<sup>o</sup>C around 1 pm. | ||
| + | |||
| + | The [http://www.ncbi.nlm.nih.gov/pmc/articles/PMC145660/pdf/240536.pdf Pope & Kent (1996)] appears to be very efficient, as the plates were full of colonies: | ||
| + | |||
| + | [[Image:10/5 Transformation Plates.jpg]] | ||
| + | |||
| + | Next time suspending the cells in water before spreading, or even making dilutions should be tried. | ||
| + | |||
| + | PCR Screening: | ||
| + | 8 colonies were picked from each transformation plate except for VP130, and suspended in 50 uL of water on a microplate. | ||
| + | |||
| + | *Plate Layout | ||
| + | **Row 1- Flu 1:1 | ||
| + | **Row 2- Flu 1:3 | ||
| + | **Row 3- OmpA | ||
| + | **Row 4- J61100 | ||
| + | |||
| + | The first two rows (Flu 1:1 and 1:3) were PCR'd by adding 1 uL of each sample to a PCR tube with a drop of mineral oil in them. A master mix was made, and 50 uL was added to each tube (should have been 49 uL). | ||
| + | |||
| + | Master Mix (18x) | ||
| + | *180 uL 5x Phusion Buffer | ||
| + | *18 uL 10 mM dNTPs | ||
| + | *45 uL VF2 | ||
| + | *45 uL VR | ||
| + | *9 uL Phusion polymerase | ||
| + | *585 uL Ultra pure H2O | ||
| + | |||
| + | PCR Program | ||
| + | *1. 95 C for 10 min | ||
| + | *2. 95 C for 30 sec | ||
| + | *3. 62 C for 30 sec | ||
| + | *4. 72 C for 2 min | ||
| + | *5. Return to step two 34 times | ||
| + | *6. 72 C for 10 min | ||
| + | *7. 4 C store | ||
| + | |||
| + | This PCR protocol was adopted from the following sources: | ||
| + | *[http://openwetware.org/wiki/Engineering_BioBrick_vectors_from_BioBrick_parts/Colony_PCR_protocol OpenWetWare BioBrick Primers Colony PCR] | ||
| + | *[http://openwetware.org/wiki/Endy:Colony_PCR OpenWetWare Endy Colony PCR] | ||
| + | *[http://www.finnzymes.com/pdf/f530sl_phusion_datasheet_2_2_low.pdf Finnzymes Phusion Manual] | ||
| + | |||
| + | Also- loosened the lids on Pseudomonas liquid cultures to provide air exchange, and returned yesterday's Pseduomonas plates to 30C incubator, without shaking. | ||
Revision as of 06:01, 9 October 2010
| Sunday | Monday | Tuesday | Wednesday | Thursday | Friday | Saturday | |
| Week 1 | - | 6/28/2010 | 6/29/2010 | 6/30/2010 | 7/1/2010 | 7/2/2010 | - |
| Week 2 | - | - | - | 7/7/2010 | 7/8/2010 | 7/9/2010 | 7/10/2010 |
| Week 3 | - | 7/12/2010 | 7/13/2010 | 7/14/2010 | - | - | - |
| Week 4 | - | - | - | - | - | - | - |
| Week 5 | 7/25/2010 | 7/26/2010 | 7/27/2010 | 7/28/2010 | 7/29/2010 | 7/30/2010 | 7/31/2010 |
| Week 6 | 8/1/2010 | 8/2/2010 | 8/3/2010 | 8/4/2010 | 8/5/2010 | 8/6/2010 | - |
| Week 7 | 8/8/2010 | 8/9/2010 | 8/10/2010 | 8/11/2010 | 8/12/2010 | - | 8/14/2010 |
| Week 8 | - | 8/16/2010 | - | - | - | - | - |
| Week 9 | - | - | - | - | 8/26/2010 | 8/27/2010 | - |
| Week 10 | 8/29/2010 | 8/30/2010 | 8/31/2010 | 9/1/2010 | 9/2/2010 | 9/3/2010 | 9/4/2010 |
| Week 11 | 9/5/2010 | - | - | - | - | - | - |
| Week 12 | 9/12/2010 | - | - | - | - | - | - |
| Week 13 | - | - | - | - | - | - | - |
| Week 14 | - | - | - | 9/29/2010 | 9/30/2010 | - | - |
| Week 14 | - | 10/1/2010 | - | - | - | - | - |
Oil Sands Lab NotebookThis team includes Ann Lesnefsky, Bryce Rajabian, Marcus Lehr, Alex Pyden, Jeremy Golden, John Seong Kyu Yang and Kilho Lee. Results to Date6/28/2010Pseudomonas putida KT2440 Antibiotic Resistance
6/29/2010Pseudomonas putida KT2440 Antibiotic Resistance 6/29/2010 and 6/30/2010Pseudomonas putida KT2440 Antibiotic Resistance 7/1/2010Literature Review Napthenic Acids Composition & HPLC Analysis 7/2/2010Pseudomonas putida KT2440 Antibiotic Resistance 7/7/2010Ann Biobrick Transformation with Alex and Jennifer
7/8/2010Ann Biobrick Transformation with Marc, Katie and Audra according to the heat shock transformation protocol
7/9/2010Ann Biobrick Transformation with Josh, Prae, Charlie according to the miniprep protocol
7/10/2010Ann Biobrick Transformation with Marcus, Bryce, Kilho, Josh, Charlie, Jeremy Miniprep
Digest
Gel
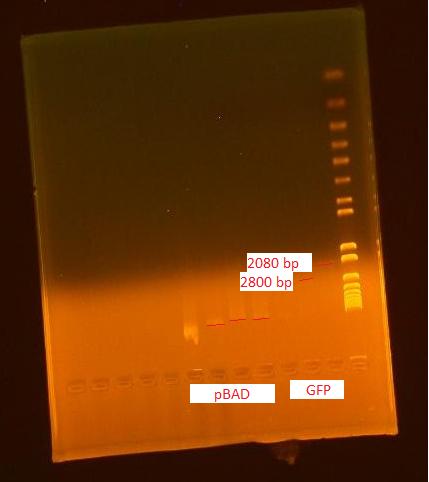
7/12/2010Ann Biobrick Transformation with Jeremy Gel
We found that we successfully extracted BBa_179015, T7-GFP, because there were expected bands at 906 and 2079 as faintly seen in the gel picture above. No appeared for biobrick Bba_179005, T7 promoter, so this biobrick will be transformed again. Biobrick Transformation take 2 with Jeremy Autoclaved DI water for transformation and autoclaved sterile containers
7/13/2010Biobrick Transformation take 2 with Jeremy, Marcus, Josh, Kevin, Audra, Katie Ann Performed according to the electroporation transformation
7/14/2010Biobrick Transformation take 2 Ann Electroporation Transformation All of the transformation plates grew out! (minus the negative control of course) This means we should do all transformations by electroporation from now on. Just check with the Lin Lab to make sure we can use the electroporation machine for a few hours before starting Miniprep
INPNC Biobrick part
7/25/2010Biobrick Transformation of suface display and pBAD Ann Tonight I started an 8mL culture of E. coli DH5alpha in LB broth and place in the 30C shaker to grow out overnight 7/26/2010Biobrick Transformation of suface display and pBAD Ann Electroporation transformation Started larger culture for comp cell preparation in 40mL of LB in a 500 mL sterile container at 9:00am At 11:30am the OD of the culture was 0.730 and the cultures were placed on ice The transformation of the biobricks was performed for according to the electroporation transformation protocol listed under the protocol section The biobricks removed from the registry are as follows
The miniprep of I79015 was used as a positive control and a negitive control was also run The OD600 of the comp cells were not measured All of the time constants for the transformation were above 5 the GFP, linker parts and the positive and negitive control were plated on 100 mg/mL AMP plates. The pBAD part and negitive control were plated on 50 mg/mL KAN 7/27/2010Biobrick Transformation of suface display and pBAD Transformation Results The GFP and linker transformation worked and many colonies grew out on the 100 mg/mL AMP plates The pBAD part did not grow out on the KAN plates. Upon further inspection this plasmid needs to be grown with IPTG to switch the origin of replication to a high copy above 100 copies per cell. Otherwise, another orgin of replication dominates that is less than 10 copies per cell and the antibiotic concentration of 50 mg/mL maybe too high. This transformation will have to be tried again Miniprep of surface display At 9:00am a 5 mL culture of the newly transformed GFP part, the newly transformed linker part, the ompA part transformed previously and the INP from the UC davis team were started for a miniprep in LB with 100 mg/mL AMP. The modified miniprep protocol was used listed on the protocol section of the wiki to get a higher DNA concentration At 9:00pm the GFP, linker and INP parts were miniprepped. The ompA culture had not grown out (b/c not enough -80C freezer stock was added) and a new culture was restarted at 11:00pm in 5 mL of LB with 100 mg/mL AMP. Pouring Plates More LB with 100 mg/mL AMP plates and LB with 25 mg/mL KAN plates were poured 7/28/2010OmpA Miniprep The OmpA culture grew out and was miniprepped according the the modified miniprep protocol at 11:30am Nanodrop of surface display parts The minipreped cultures from yesterday were nanodropped to determine the DNA concentration according to the protocol in the protocol section of the wiki
Digest of surface display parts The following biobricks were digested according to the protocol on the wiki with the enzymes listed below
Gel of Digest for surface display parts A gel was run of the above digest with uncut plasmid as a control
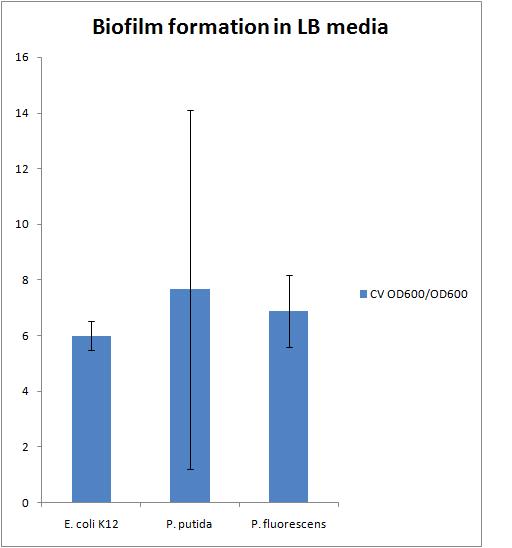
7/29/2010Biofilm Assay in LB media Started cultures of E. coli K12, P. putida (oilsands) and P. fluorescens (oilsands) in 2 mL of LB and grew in the 30C shaker overnight 7/30/2010Biofilm Assay in LB media Today we carried out the following biofilm assay protocol
The results of this experiment are presented in the chart below There is a large error in the P. putida strain because there was the largest variation in the OD 600 of the liquid culture which also resulted in a large variation in the CV OD 600 reading We need to find a reference to compare our biofilms to. This could be either a strain that does not form biofilms or a strain that forms biofilms very well Transformation of pBAD Marcus and Alex tried the transformation of pBAD again according to the electroporation protocol with Ann's Notes The recovery after electroporation was performed in 1 mL of LB with 1mM of IPTG added in order to have the pBAD part have a high copy number 30 uL of 1M IPTG solution was added to a 25 mg/mL KAN plate and spread with sterile beads in order to keep the high copy plasmid in the pBAD part while plating 7/30/2010Transformation of pBAD The transformation was successful and there were about 30 colonies on the plate. One colony was picked at 7:30 pm for a miniprep the next day and added to 5mL of LB + 25 mg/mL KAN + 1 mM IPTG and grown out in the 30C shaker Another culture was started of GFP from the -80C freezer stock in 5 mL of LB + 100 mg/mL AMP for a miniprep. Both the ligations with GFP did not work, so as long as we are miniprepping and digesting we want to re-verify this part 8/1/2010Nanodrop of pBAD and GFP verification The miniprep was performed for the two cultures started last night according the the MODIFIED miniprep protocol. A frozen stock was made of the pBAD biobrick and placed in the -80C freezer The DNA concentrations reported by the nanodrop are as follows
The pBAD grew out very dense and when IPTG is added the copy number is over 100 so the DNA concentration was very high Digest of pBAD and GFP verification The parts were digested according to the protocol in the protocol section of the wiki for a 50 uL reaction volume
8/2/2010Gel of Digested Parts
We did not get the pBAD part potentially because the miniprep culture overgrew and the cells started to lyse their chromosomal DNA (resulting in extremely long bands in the gel). We will try miniprepping the DNA from this culture again letting the culture grow out for a shorter amount of time before the miniprep flu primers Primers were ordered today to amplify the flu operon of E. coli K12 by colony PCR 8/3/2010Minimal Media Recipes Minimal media was mixed today according to the following recipes (also found in the media recipe section of the notebooks) Culturing Bacteria in Naphthenic Acids (NA's) The following 9 cultures were started to test bacteria growth in NA media
1 mL of the above media was added to a 15 mL falcon tube and innoculated with -80C freezer stock of each culture and grown in the 30C shaker (psuedomonas strains are temperature sensitive). The cultures were started at 7:30 pm 8/4/2010Culturing Bacteria in Naphthenic Acids (NA's) At 12:30 pm the OD600 of the cultures started yesterday were measured and are listed below. These results must be taken with a grain of salt because the cultures were started from the -80C freezer stock with different amounts of innoculum
P. flourescens oilsand seems to love this medium and grew out very dense! P. putida oilsand showed signs of starting to grow. E. coil K12 did not grow at all and dead cells pellets appeared on the bottom of the culture flask From these results we can go ahead and start cultures for the biofilm assay experiment, and time the cultures so they all become dense on around the same day. If all cultures are started 24 hours in advanced they all should saturate by the next day. The E. coli K12 and P. putida oilsands cultures were placed back in the 30C shaker to keep growing. By 9:30pm the P. putida oilsands cultures had saturated and the E. coli K12 cultures had still not grown As a control for the biofilm assay E. coli K12 will be grown in M9 minimal media with glucose as a carbon source with and without casamino acids. We will not attempt to grow E. coli K12 in Bushnell-Haas media. Measuring pH of Bushnell-Haas minial media with cyclohexane carboxylic acid adjusted to pH 9 Today Marc showed us how to use the pH meter in the Lin Lab. Yesterday the pH of the media was estimated by pH paper to be between 8 and 9. The actual pH of the media is 7.35. The pH of the already adjusted media from yesterday was increased by added more 250 uL of 0.1 M NaOH to 5mL of BH CHCA media and the pH ready 7.6 using the pH probe. Another 250 uL of NaOH was added to this mixutre and the pH read 8.8 using the pH probe. pH paper was dotted using this mixture to see the exact color it should turn when the pH is close to 9 (the paper looks more blue than green when the dot first appears).
Bacterial Growth in pH of 9 After determining that 1mL of the supposedly adready adjusted BH-CHCA media needed 100 uL of 0.1 M NaOH soultion per mL to really be close to a pH of 9, new overnight cultures were started of the two pseudomonas oilsand strains in the 1 mL of the BH-CHCA pH adjusted media from yesterday adding an additional 100 uL of 0.1 M NaOH. These two cultures were placed in the 30C shaker to grow out overnight at 8:30 pm.
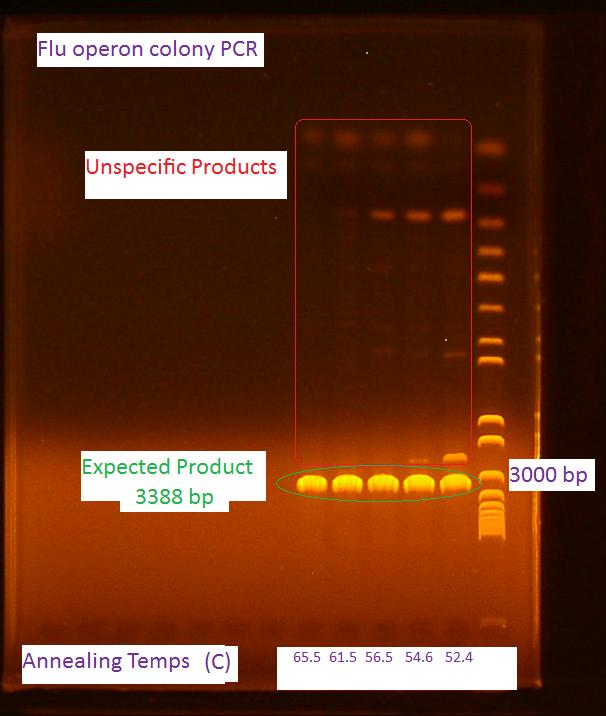
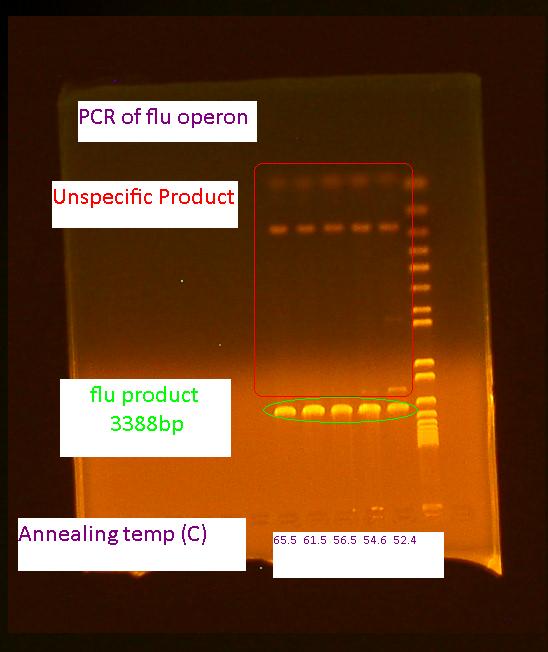
Miniprep of pBAD take 2 At 10:45 pm a 5 mL culture of the pBAD biobrick was started from the -80C freezer stock in LB + 25 mg/mL KAN + 1 mM IPTG for a miniprep tomorrow morning PCR of flu operon of E. coli K12 The primers ordered on Monday came in today! PCR reagents were picked up from the life science store
The PCR was run according to the following colony PCR protocol The final annealing cycle for 10 minutes at 72 C was accidentally added in the last 30 cycle loop. This mistake was caught after 3 cycles and the PCR was stopped, the program fixed and the reaction restarted. Gel of flu colony PCR The following gel was run in a 0.7% agarose gel at 85V according to the protocol on the wiki For the first 5 cycles the annealing temperature was lowered to the Tm of the 16 or 18 bp that was complementary to the E. coli K12 template. A gradient of temperature was run for the annealing temperatures of the first five cylces as labeled in the gel 8/5/2010Bacterial Growth at pH 9 At 9:00am the two cultures started in the BH-CHCA with the pH actually adjusted to around 9 was checked. Both P. putida oilsands and P. fluorescens oilsands showed slight signs of growth. Later in the day at3:30 pm the cultures are denser were denser and the OD600 of the culture is:
It should be noted some of the salts precipitated out of solution in the 1 mL cultures Miniprep of pBAD take 2 The miniprep was started at 8:45am. After 10 hours of growth the culture was saturated and the miniprep started according to the modified miniprep protocol. Nanodrop of pBAD take 2 The concentration of the pBAD promoter miniprepped earlier today is 418 ng/uL. It is most likely still contaminated with this high concentration but we will digest it and run a gel to check anyways Digest of pBAD take 2 the digest was performed according to the digest protocol in the protocol section of the wiki pBAD #3 was digested with EcoRI and SpeI pBAD #4 was digested with SpeI and PstI PCR of flu operon The PCR of the flu operon was repeated 3 more times at higher annealing temperatures because this showed to have less unspecific product (shown by the gel from yesterday)according to this modified colony PCR protocol. The volume of the reactions were increased to 50 uL to do a PCR purification step afterwards. Gel of flu operon PCR and pBAD promoter digest The gel ran exactly the same for the pBAD promoter as on 8/2/2010. The sample is probably contaminated and a new colony should be picked from the transformation plate and screened. Also, no bands appeared for the flu PCR. One error might be because we did not include an initial cell lysis step. The PCR will be tried again tomorrow Biofilm assay in minimal media Cultures were started according to the following biofilm assay protocol to inoculate the microplate for the biofilm assay tomorrow. 8/6/2010PCR of flu take 3 The first PCR of the flu operon was repeated with an added cell lysis step according to the following modified protocol Gel of PCR of flu take 3 Invitrogen 1 kb plus ladder Due to the unspecific product the flu operon will be purified with gel purification Biofilm Assay The OD600 of the cultures started yesterday are listed below along with the volume for resuspension after 1 mL of culture was pelleted:
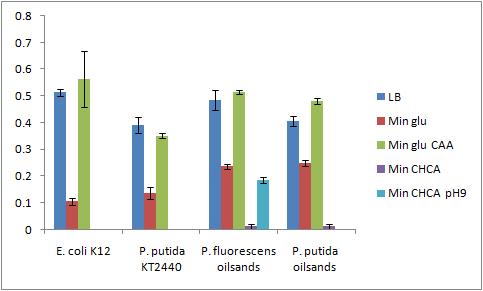
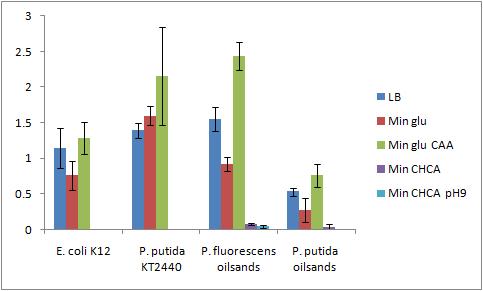
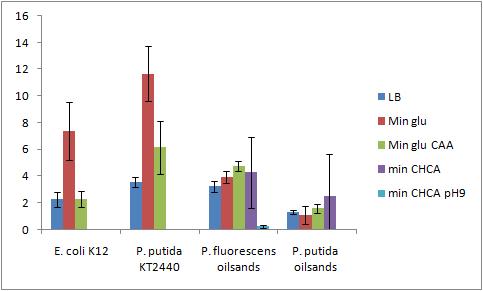
Not all of the cultures grew out (P. putida KT2440 Bushnell-Haas media plus cyclohexane carboylic acid, P. putida KT2440 Bushnell-Haas media plus cyclohexane carboylic acid pH 9, P. putida oilsands Bushnell-Haas media plus cyclohexane carboxylic acid pH 9) The modified bio assay protocol was used to inoculate the microplate The microplate was inoculated at 4:30pm 8/8/2010Biofilm Assay The washing and CV staining of the bioassay was performed according to the modified protocol on 8/6/2010
The Crystal Violet OD 600 after staining is show in the following graph
The Crystal Violet OD 600 to OD 600 after growth ratio is shown in the following graph
A strong conclusion cannot be drawn about the biofilm forming abilities in minimal media with cyclohexane carboxylic acids (CHCA) because the cells did not grow out very dense. This is why the error bars are large. It is also interesting that P. fluorescens in pH 9 did grow out but did not form biofilms even though the cell culture grew relatively well. We need a negitive control though to confirm this result 8/9/2010PCR of flu for gel extraction Because there are unspecific products from the PCR of flu, a gel extraction will have to be used to isolate the desired DNA 50 uL reaction volume for PCR was performed using 65.5C for the initial annealing temperature as shown in the following colony PCR protocol of flu. The higher annealing temperature reduces the unspecific products Gel Extraction of flu operon A gel extraction was performed according to the following gel extraction protocol 20 uL of the flu #14 PCR was mixed with 5 uL of blue juice to run the gel The weight of the eppendorf tube was 1.0284 g The weight of the eppendorf tube with the sample was 1.2030 g The weight of the gel sample was 0.175 g 524 uL of QG buffer was added to disolve the gel sample No DNA appeared on the nanodrop, Marc ran the flu gel purified sample on his gel to confirm no DNA eluted DNA did appear on the gel that Marc ran so we do have DNA from the gel extraction. The nanodrop may not have been able to pick it up because it was too low of a concentration or it was contaminated (very high 260/230 reading) 8/10/2010Ann and Bryce Transformation of Constituative Promoter and KAN backbone The transformation was performed according to the electroporation protocol in the protocol section of the wiki The comp cell culture was started at 9:10am by adding the 8 mL overnight culture into 40mL of LB media in a 500 mL sterile flask The OD600 of the culture was checked at 11:20am and was 1.0. This culture was slightly overgrown but was immediately placed on ice and used anyways (this might lower the efficency of the procedure). The biobricks we are transforming today are:
The transformation was done at 2:15pm and the cells were plated on the following plates
and placed in the 37C incubator to grow out overnight We plan on ligating the flu operon into the KAN backbone than cutting than inserting the promoter in front of this part. We have to take this approach because the flu operon cannot be digested with PstI. Marcus Brought over 5 mL of each standard pH buffer (4, 7, 10) from Lin lab. Started NA extraction from oil sand ore:
See the NA Extraction Protocol. 8/11/2010Miniprep of constitutive promoter and backbone with KAN resistance The transformation was successful and both plates with the constitutive promoter and the backbone with KAN resistance grew up (even though the positive control did not grow...) The constitutive promoter part is followed by RFP. We saw this on the transformation plates because the colonies were red. A colony was picked from each plate and at 9:00am and 5mL cultures were started in the following media:
These cultures were placed in the 37C incubator to grow up at 9:00pm the cultures were minipreped according to the modified miniprep protocol Nanodrop of constitutive promoter and KAN backbone The concentration of the minipreps are as follows:
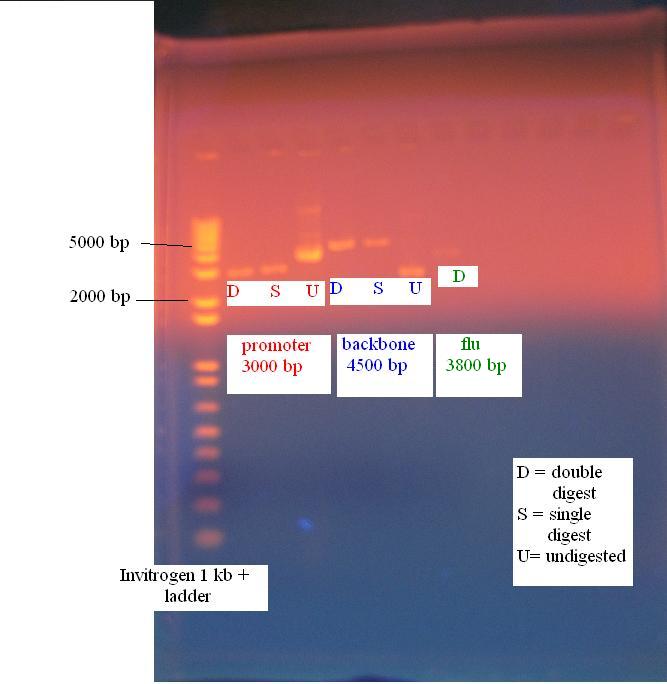
PCR of flu operon from the gel extraction product Using gel extraction product for ligations lowers the efficency of the ligation. To get around this problem we performed another PCR reaction using the gel extraction product as the template according to the following PCR protocol Gel of PCR of flu operon from gel extraction product There was more unspecific product from trying the PCR this reaction from the gel extracted part, so the gel extracted part will be used for further genetic manipulations 8/12/2010Digest of constitutive promoter, KAN backbone and flu operon The following digests were performed
All digests except for the KAN backbone digested with EcoRI and SpeI were 20 uL volumes. 1 uL of each enzyme was added along with 0.5 uL of BSA to the 20 uL reactions and the rest of the reagents were ajusted by multiplying the 50 uL volume by 0.4. To make up for the extra volume of BSA and enzymes, the extra amount was subtracted from the amount of water added. Because the KAN backbone digested with EcoRI and SpeI is going to be PCR purified, double the amount of DNA was added and a 50 uL volume reaction was performed Since no nanodrop reading was made for the flu operon, no water was added and only the gel extraction was used Gel of constitutive promoter, KAN backbone and flu operon All parts digested accordingly and are ready for the ligation PCR purification of KAN backbone The PCR purification was performed with the 50 uL digest of the KAN backbone with EcoRI and SpeI according to the protocol in the protocol section of the wiki. 250 uL of buffer PB was used to mix with the digest before adding the mixture to the column Nanodrop of PCR purified KAN backbone The PCR purified KAN backbone had a concentration of 14.8 ng/uL with a 260/280 ratio of 1.86 and a 260/230 ratio of 0.91. These ratios indicate that the sample is contaminated but there is still a high enough peak at 260 to show DNA is there. Another gel could be run to verify that DNA is there, but this step will be skipped and the DNA will be used for the ligation Ligation of flu operon with the KAN backbone The ligation was performed according to the following T4 ligase protocol Marcus Removed ore/NaOH extraction mixture from shaker. Poured liquid into 50 mL tube, and strained the solids through a coffee filter and funnel to collect as much liquid as possible. Recovered about 20 mL. This was filtered through a 50 mL Steriflip vacuum tube, however, the filter became clogged and a second one was used, which required an extended vacuum time (in the future the mixture should be centrifuged first for several minutes). Recovered a total of about 40 mL (???). 8/13/2010Marcus
8/14/2010Transformation of the Flu Operon + Kan backbone ligation The Heat shock transformation protocol was used E.Coli DH5alpha was used as competent cells and had a prewashing OD600 of .845 and a post washing OD600 of .787. Two seperate transformations were perfromed both with 100 uL Comp. Cells + 10 uL Flu + KAN BB Ligation Both transformations were plated on LB+Kan+IPTG(30 uL) and are currently incubating at 37 C in the ERB. 8/16/2010Transformation of Flu Operon + Kanamycin Backbone Ligation
Both plates were left to incubate longer because the slow growth may be due to the high concentration of Kanamycin (50mg/mL) added to the plates. 8/26/2010Overnight Cultures of the Flu Operon + Kanamycin Backbone Transformation Colonies appeared on both transformation plates. Overnight cultures were started from both plates in 5 mL LB + KAN 50 + 1 mM IPTG. Marcus Attempted to perform electroporation according to Electroporation Protocol 2 with parts:
However, cells took too long to grow out because only 1 mL of overnight was used to inoculate the 100 mL fresh culture. OD600 was only 0.142 after 4.5 hours. Made 10 Amp and 10 LB plates. 8/27/2010Overnight Cultures of the Flu Operon + Kanamycin Backbone Transformation Take 2 The first set of overnight cultures are experiencing slow growth. A second set of overnight cultures were started in lower Kanamycin levels (5 mL LB + KAN 25 + 1 mM IPTG). 8/29/2010Miniprep & Nanodrop of Flu + Kan Backbone Ligation Before the miniprep .5 mL of both cultures were used to make a frozen stock. The frozen stocks now reside in the -20 C freezer in the ERB. The first set of overnight cultures were miniprepped and eluted in EB Buffer. After Nanodropping the DNA Concentrations for plated #1 and #2 were 9.5 and 4.0 ng/uL, respectively. 8/30/2010Digest of Flu + Kan Backbone Ligation The miniprepped cells (from 8/29/2010) were digested with EcoRI & Spel. (To screen if the ligation worked properly or if mixed sites formed). 20 uL of DNA from ligation #1 and 25 uL of DNA from ligation #2 were used for the digest (to compensate for low miniprep yields).
Marcus and John Repeated electroporation attempted on 8/26 using Electroporation Protocol 2. Parts:
We didn't quite have enough comp cells, because we forgot to account for controls when making the cells, so a couple samples were a bit short (22L and another). 8/31/2010Gel of Flu + Kan Backbone Ligation A Gel was ran with the two digested ligations (from 8/30/2010) and failed to illuminate under UV light because of a mistake in the EtBr concentration used (2 mg/mL was used instead of the standard 10 mg/mL). Miniprep of Flu Ligation #1 & #2 (2nd Set) and 2M The Nanodrop values are as follows:
9/1/2010Gel of Flu + Kan Backbone Ligation (Take 2) A Gel was ran with the same two digested ligations (from 8/30/2010) and failed to illuminate under UV light. The source of error is believed to be that the DNA ran off the gel because the voltage was originally set at 85V (to run for 1.5 hrs) and upon returning (after 1.5 hrs) the voltage had been changed to 105V (by another lab member roughly 15 minutes into the start of the gel). 9/2/2010Digest of Flu + Kan Backbone Ligation (Take 2) The miniprepped cells (from 8/31/2010) were digested with Xba1 (To screen if the ligation worked by checking for a band at 7790 base pairs). Ann's digest calculator was used to calculate the proper amount of DNA to use. Miniprep of 22L, 13A, and Kan Backbone The DNA concentrations from the Miniprep by Nanodropping are as follows:
9/3/2010Overnight cultures of Flu Ligation 1 & 2 (set 3), KAN BB, 2M The Flu ligations and KAN Backbone cultures were started in 5 mL of LB + KAN 25 + 1 mM IPTG The 2M culture was started in 5 mL of LB + AMP 9/4/2010Miniprep of of Flu Ligation 1 & 2 (set 3), KAN BB, 2M The DNA concentrations from the Miniprep by Nanodropping are as follows:
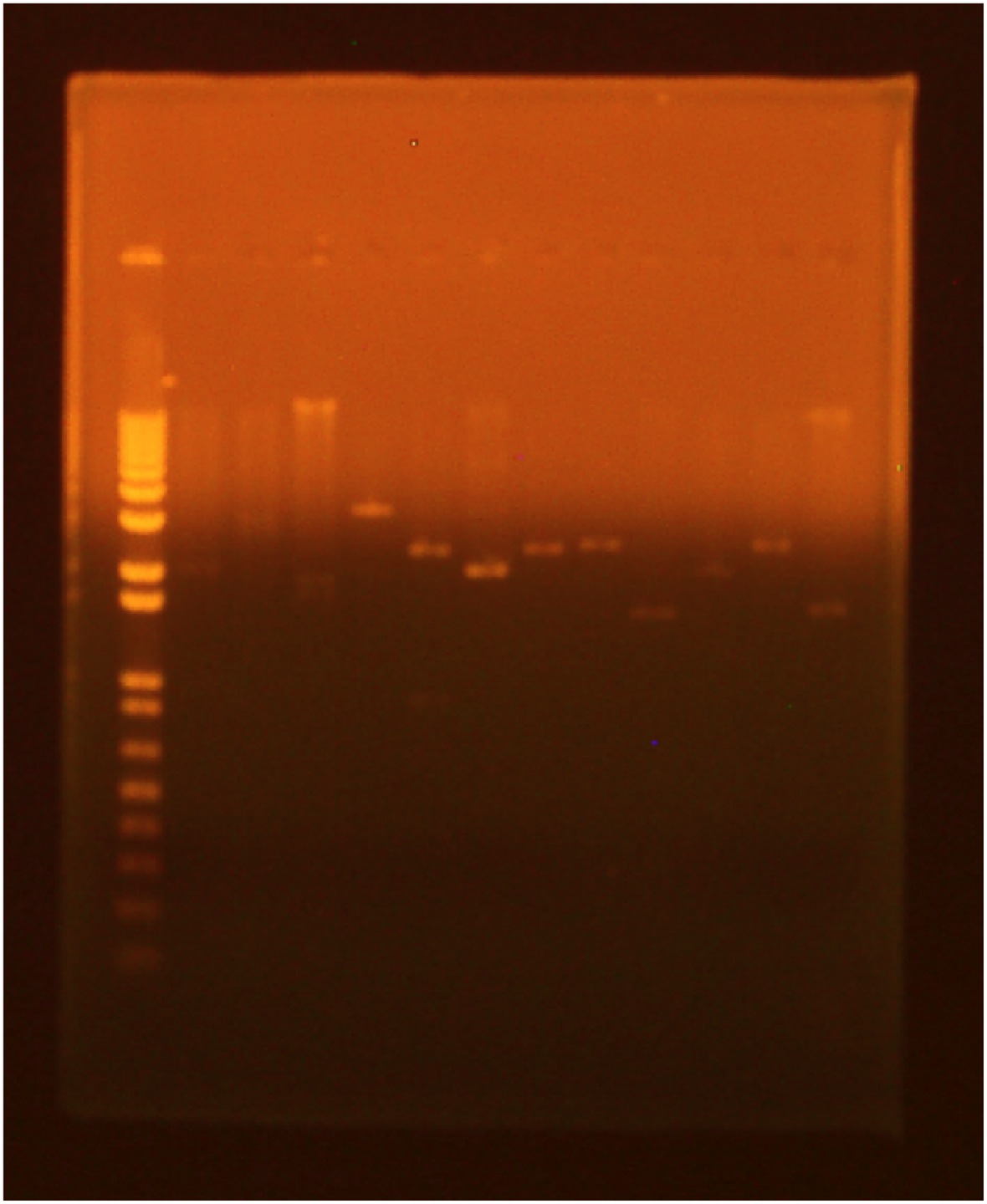
9/5/2010Gel of Flu Ligation Transformation (take 3)
|
In the Lab |
Marcus
Ore extracts were combined into a single tube and re-filtered, then stored in acid storage cabinet.
9/11/2010
Marcus and John
Made dilution protocol for CHCA starting with 1500 mg/L stock solution made by Ann previously. Made 500, 250, 125, 50, 10, 5, and 1 mg/L solutions, with at least 0.75 mL left of each after dilutions. These were stored in the fume hood and then given to Mike for analysis on the HPLC.
Also, autoclaved liquid waste.
9/12/2010
Alex
Made E. coli K12 broth culture from plate
- 2 mL LB in 15mL tube
- 37°C, 200 rpm shaking - ERB 1230
Uploaded NanR cloning protocol
9/24/2010
Marcus, Bryce, and Ben
Ran PCR of Flu operon according to the NanR cloning PCR protocol, with modifications as follows:
- 1x PCR Mix:
- 36.25 uL Ultra pure H2O
- 10 uL 5x Phusion Buffer
- 1 uL 10 mM dNTPs
- 0.625 uL Forward Flu Primer
- 0.625 uL Reverse Flu Primer
- 1 uL DNA Template (1:10 O/N dilution)
- 0.5 uL Phusion polymerase
- PCR Program (FLUPCR)
- 1. 96 C for 6 min
- 2. 98 C for 10 sec
- 3. 65.5 C for 30 sec
- 4. 72 C for 1 min 45 sec
- 5. Return to step 2 four times
- 6. 98 C for 10 sec
- 7. 67 C for 30 sec
- 8. 72 C for 1 min 45 sec
- 9. Return to step six 29 times
- 10. 72 C for 10 min
- 11. 4 C store
Unfortunately, a math mistake was made while making the master mix, which was discovered due to having too much master mix left over. The volumes used to create the master mix were:
- 181.25 uL Ultra pure H2O
- 100 uL 5x Phusion Buffer
- 10 uL 10 mM dNTPs
- 6.25 uL Forward Flu Primer
- 6.25 uL Reverse Flu Primer
- 10 uL DNA Template
2.5 uL Phusion polymerase
9/27/2010
Marcus
Got some chloramphenicol from the Lin lab (34 mg/mL 1000x stock) and made 10 chloramphenicol plates and 10 LB plates. Also, made 45 mL of a LB+CM34 stock, and autoclaved 500 mL of LB.
9/28/2010
Marcus
Ran gel of Flu PCR product. Used the Gel Electrophoresis protocol with the following variations:
- Doubled PCR product volume, in case gel extraction was needed
- 9.6 uL PCR product, 2.4 uL Blue Juice
- Ran at 110V for 50 min
It appears samples 3 and 4 were successful, however the gel needs to be re-run because of excessive smearing. Jeremy Minty suggested lower voltage and less time. Also, "nonspecific" bands seen by Ann and Bryce were nearly nonexistent and also seen in lanes 1 and 2, suggesting that they are not nonspecific product, but something else, such as primer-dimer.
9/29/2010
Alex and John
Dissolved M9 salts to 50X
- All of component A in 1000 mL DIwater
- All of component B in 1000 mL DIwater
Autoclaved both: each split into two 1000mL bottles filled w/ 500mL each (four bottles total)
- 30 min sterilize time
Began biofilm assay following Alex's biofilm assay protocol
Made broth cultures of P. putida LD1, P. fluorescens LD2, and LD1+LD2 coculture from plates, and P. putida KT2440 from Lin Lab frozen stock
- KT2440 inoculated into 2 mL LB in 15mL tube
- others inoculated into 2 mL LB + 100μg/mL amp in 15mL tube
- all incubated in 30°C, 200rpm shaking -- ERB 1230, 8:45pm
Streaked KT2440 on LB plate from frozen
- incubated 37°C -- ERB 1239
Added KT2440 to strain database Mixed M9 A and B solutions and diluted 1/50 in 200 mL sterile DIwater -- in 4 50mL tubes
- each tube 1 mL M9A, 1 mL M9B and 48 mL water
Alex
Uploaded Alex's biofilm assay protocol
Added borax to two of the M9 tubes (100 mL) to buffer at ~pH 9.2
- (.01 mol/L)*(.05 L)*(381.37 g/mol) = 190.7 mg borax/50 mL water
- Tested pH with pH indicator strips
- ~9
- Vacuum-filtered 100 mL w/ steriflips
Marcus
Repeated Flu PCR Gel from yesterday, with the following variations:
- Regular amount of product/loading dye (4.8 and 1.2 uL)
- Ran at 86V for 60 min
Results look much better, bands are tighter, and conclusions are the same as yesterday. No gel extraction will be necessary.
9/30/2010
Alex
Aliquot M9 and buffered M9 into five 15mL tubes each Need 15 mL stocks of each of the following media -- each 15 mL neutral and 15 mL buffered at pH 9:
- M9
- M9 + 0.4% glucose
- M9 + Acros NAs (250mg/L)
- M9 + Sigma Aldrich NAs (250mg/L)
- M9 + both Acros & Sigma NAs (each 250mg/L)
- LB
Added 150 μL 40% glucose to appropriate tubes (.4% final concentration) Aliquot 15 mL LB into each of two 15mL tubes Added NAs to appropriate tubes
- Acros: (.25 mg/mL)*(15mL)/(.91 mg/μL) = 4.12 μL - Sigma: (.25 mg/mL)*(15mL)/(.92 mg/μL) = 4.08 μL
- added 4.1 μL of each NA to each respective tube
Added borax to one tube of LB to buffer at pH9
- (190.7 mg)*(15μL/50μL) = 57.2 mg per 15mL LB
- tested pH with indicator strip
- ~9
Tested pH of neutral M9 with indicator strip
- ~7
Moved KT2440 LB plate from 37°C to 4°C -- ERB 1239
Continued Alex's biofilm assay protocol
- made one 96-well plate for all strains and media at pH7 only
- plate template :
- 100 μL/well: 99 μL medium + 1 μL culture
- covered plate with gas-permeable membrane
- Started 24-hr kinetic reading in Lin Lab microplate reader
- 30°C
- OD600 absorbance
- read every 30 min
Made broth cultures for LD1, LD2 and KT2440 from yesterday's overnight broths
- 2 mL each in a 15mL tube
- 30°C, 200 rpm shaking - 6:20pm
Discarded yesterday's overnight broths
Marcus
Purified Flu PCR product with Qiagen PCR purification kit, and eluted in ultra pure H2O instead of EB. 35 uL remaining from samples 3 and 4 of PCR were combined and purified, and stored at 4 C.
Bryce came in in the evening and got the concentration of the purified samples, along with some minipreps according to the Nanodrop protocol:
Flu PCR Product- 29.8 ng/uL 13A (pSB1AT3)- 240.5 ng/uL 22L (BBa_I13504)- 467.4 ng/uL 2M (B0034)- 661.4 ng/uL
Bryce also digested the Flu operon and pSB1AT3 with EcoRI and SpeI according to the DNA Digest protocol. He used 17 uL of the Flu PCR product and 5 uL of pSB1AT3.
10/1/2010
Alex
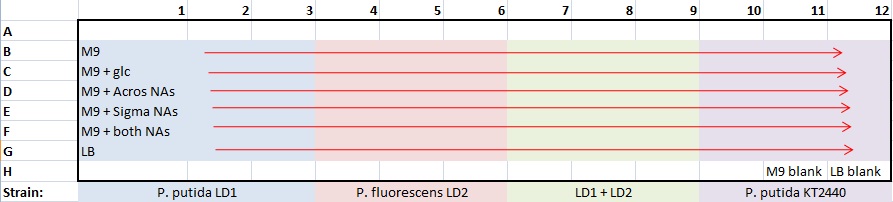
Moved broth cultures 37°C to 4°C -- 1:30pm (19-hr culture) Prepared biofilm assay plate for all pH9 tests - following Alex's biofilm assay protocol
- plate template:
- 100 μL/well: 99 μL medium + 1 μL culture
- stored plate in Lin Lab 4°C -- 2:20pm
Saved data for curves from ph7 plate Read pH7 plate ODs Performed biofilm assay on ph7 plate Started 24-hr kinetic reading for pH9 plate -- ~midnight
Discarded pH7 plate
Processed pH7 plate data
- uploaded results here
Uploaded pH7 kinetic data here
10/3/10
Alex
Ran CV biofilm assay on pH9 plate, following my protocol
- discarded plate
- uploaded results
- uploaded pH9 kinetic raw data
10/4/2010
Marcus and Ben
Performed ligation of Flu operon and pSB1AT3 according to the Ginko Bioworks Ligation protocol, with modifications.
- Calculated amount from digests to add to ligation for 1:1 and 3:1 insert:vector ratios
- Amount of DNA in digests:
- Flu PCR- 29.8 ng/uL x 17 uL= 506.6 ng / 50 uL = 10.132 ng/uL
- pSB1AT3- 240.5 ng/uL x 5 uL= 1202.5 ng / 50 uL = 24.05 ng/uL
- Used an online calculator to find moles:
- Flu PCR- 230.81 fmol/50 uL
- pSB1AT3- 537.68 fmol/50 uL
- This gives a molar ratio of 0.4293
- Amount of DNA in digests:
1:1 Ligation Mix
- 4 uL Flu PCR x 10.132 ng/uL= 40.528 ng
- 1.7 uL pSB1AT3 x 24.05 ng/uL= 40.885 ng
- 2 uL T4 Buffer
- 1 uL T4 Ligase
- 11.3 uL Ultra pure H2O
3:1 Ligation Mix
- 2 uL Flu PCR x 10.132 ng/uL= 20.264 ng
- 2.57 uL pSB1AT3 x 24.05 ng/uL= 61.809 ng
- 2 uL T4 Buffer
- 1 uL T4 Ligase
- 12.43 uL Ultra pure H2O
Note that the conversion factor for the 3:1 ligation mixture was applied in reverse, and thus it was actually a 1:3 insert:vector ratio.
Ligation program was the same as used in the Ligation protocol.
10/5/2010
Marcus
Performed transformation according to [http://www.ncbi.nlm.nih.gov/pmc/articles/PMC145660/pdf/240536.pdf Pope & Kent (1996)].
Transformed Parts
- Flu/pSB1AT3 1:1 ligation
- Flu/pSB1AT3 1:3 ligation
- VP130
- J61100
- OmpA
- Cell only negative control
Procedure
- 2 uL of DNA was pipetted into PCR tube on ice and transferred to Lin lab
- Comp cells were thawed on ice and 50 uL was pipetted into each tube with a chilled pipette on ice
- Cells were pipetted up and down to mix throughly
- Cells were kept on ice and brought back to ERB
- Cells were plated directly onto pre-warmed plates with appropriate antibiotics
- Kevin put plates in incubator several hours before hand
It should be noted that two 200 uL aliquots of cells had to be thawed, and one was labeled "Thawed" and returned to the -80 C. Thawing should be avoided if possible.
Also, made two 5 mL LD1/LD2 co-cultures in M9+Sigma and Acros NAs, pH 7 and pH 9. Also made 2 mL pure cultures at pH 7. All culture tubes were stored in the 30 C. These cultures were made to assay growth, make new stock plates, and determine if colony morphology can be used to distinguish between species in a co-culture.
10/7/2010
Marcus
Ben moved transformation plates to 4 C yesterday, control plates were blank, and all plates except for J61100 appeared to have colonies. The Flu/pSB1AT3 ligation plates had very small colonies, so they were returned to the incubator with the J61100 plate. Also, it could be seen that many colonies on the 1:3 ligation plate appeared red, indicating that the vector had preferentially reformed. This could be because of the ratio, but dephosphorylation treatment should be used in the future for ligations.
OD600's were taken of the Pseudomonas cultures (46 hours)
- Putida pH 7- 0.157
- Fluorescens pH 7- 0.129
- Co-culture pH 7- 0.188
- Co-culture pH 9- 0.232
Surprisingly, they seem to grow better at pH 9, at least in co-culture. As expected, the co-culture did better than pure cultures. This lends some support towards Alex's biofilm assay results, however, it should still be repeated. Also, plated a serial dilution of the pH 9 co-culture, at 10-5, 10-6, and 10-7 dilutions to determine the conversion factor between OD600 and cell count. Made streak plates of all other cultures, pure cultures for stock, and pH 7 co-culture to determine if colonies could be distinguished. All cultures and plates were returned to the 30oC incubator, without shaking.
10/8/2010
Marcus
Kevin removed the transformation plates which were placed back in the incubator to the 4oC around 1 pm.
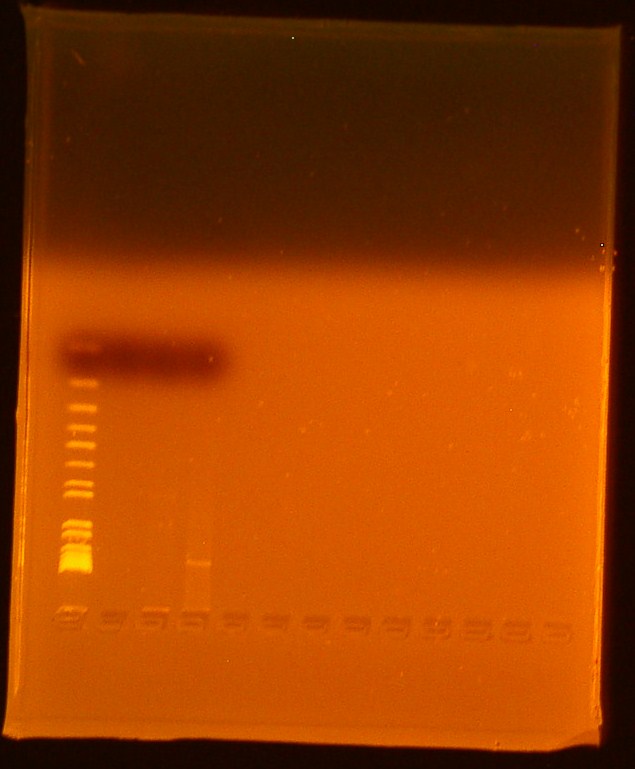
The [http://www.ncbi.nlm.nih.gov/pmc/articles/PMC145660/pdf/240536.pdf Pope & Kent (1996)] appears to be very efficient, as the plates were full of colonies:
File:10/5 Transformation Plates.jpg
Next time suspending the cells in water before spreading, or even making dilutions should be tried.
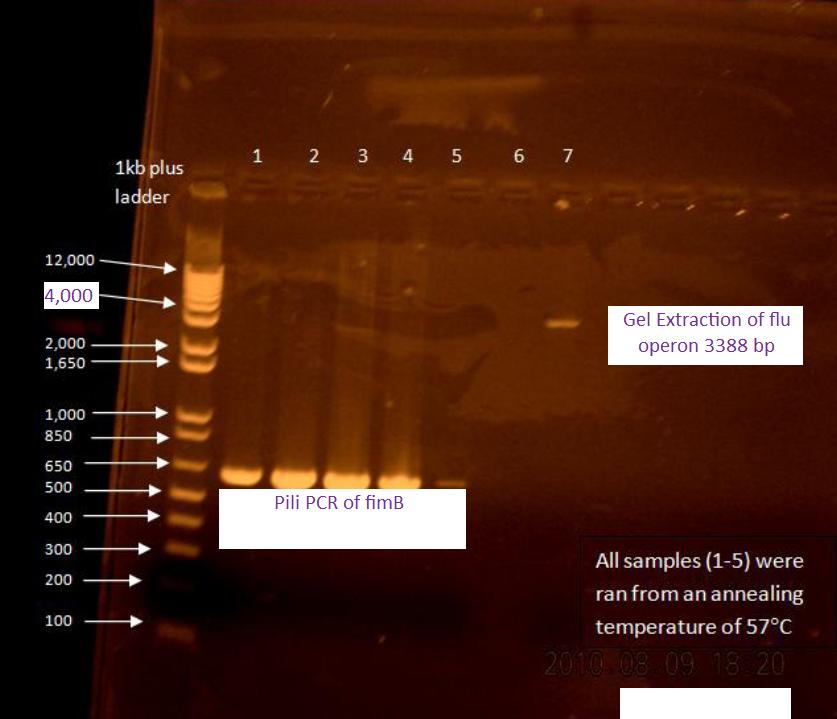
PCR Screening: 8 colonies were picked from each transformation plate except for VP130, and suspended in 50 uL of water on a microplate.
- Plate Layout
- Row 1- Flu 1:1
- Row 2- Flu 1:3
- Row 3- OmpA
- Row 4- J61100
The first two rows (Flu 1:1 and 1:3) were PCR'd by adding 1 uL of each sample to a PCR tube with a drop of mineral oil in them. A master mix was made, and 50 uL was added to each tube (should have been 49 uL).
Master Mix (18x)
- 180 uL 5x Phusion Buffer
- 18 uL 10 mM dNTPs
- 45 uL VF2
- 45 uL VR
- 9 uL Phusion polymerase
- 585 uL Ultra pure H2O
PCR Program
- 1. 95 C for 10 min
- 2. 95 C for 30 sec
- 3. 62 C for 30 sec
- 4. 72 C for 2 min
- 5. Return to step two 34 times
- 6. 72 C for 10 min
- 7. 4 C store
This PCR protocol was adopted from the following sources:
- [http://openwetware.org/wiki/Engineering_BioBrick_vectors_from_BioBrick_parts/Colony_PCR_protocol OpenWetWare BioBrick Primers Colony PCR]
- [http://openwetware.org/wiki/Endy:Colony_PCR OpenWetWare Endy Colony PCR]
- [http://www.finnzymes.com/pdf/f530sl_phusion_datasheet_2_2_low.pdf Finnzymes Phusion Manual]
Also- loosened the lids on Pseudomonas liquid cultures to provide air exchange, and returned yesterday's Pseduomonas plates to 30C incubator, without shaking.
 "
"