Team:Kyoto/Notebook
From 2010.igem.org
(Difference between revisions)
(Tuesday, July 20) |
|||
| Line 57: | Line 57: | ||
*Category: Transformation, PCR, Lysis Cassette | *Category: Transformation, PCR, Lysis Cassette | ||
|- | |- | ||
| - | |Cultured plates in which colonies was observed at 37℃ from 07/21 20:50 to 07/22 17:00. | + | |(1) Cultured plates in which colonies was observed at 37℃ from 07/21 20:50 to 07/22 17:00. |
|- | |- | ||
| - | |Made a master plate of the above plates. | + | |(2) Made a master plate of the above plates. |
|- | |- | ||
| - | |Retried Transformation of iGEM Parts. | + | |(3) Retried [[Team:Kyoto/Protocols#Transformation|Transformation]] of iGEM Parts. |
{| class="experiments" | {| class="experiments" | ||
|Name||Well<sup>*1</sup>||Sample (µl)||Competent Cells (µl)||Total (µl)||Plate||Incubation||Result | |Name||Well<sup>*1</sup>||Sample (µl)||Competent Cells (µl)||Total (µl)||Plate||Incubation||Result | ||
| Line 71: | Line 71: | ||
* *1 "1-5-G" means well 5G in [http://partsregistry.org/Help:Spring_2010_DNA_distribution Spring 2010 DNA Distribution Kit] Plate 1. | * *1 "1-5-G" means well 5G in [http://partsregistry.org/Help:Spring_2010_DNA_distribution Spring 2010 DNA Distribution Kit] Plate 1. | ||
|- | |- | ||
| - | |PCR PCR for S-R-Rz/Rz1 and S | + | |(4) [[Team:Kyoto/Protocols#PCR|PCR]] for S-R-Rz/Rz1 and S |
* Dilute λDNA (0.5µg/µl) 100 times with MilliQ. The final concentration of template λDNA was 5ng/µl. | * Dilute λDNA (0.5µg/µl) 100 times with MilliQ. The final concentration of template λDNA was 5ng/µl. | ||
{| class="experiments" | {| class="experiments" | ||
| Line 105: | Line 105: | ||
*Category: Lysis Cassette, parts | *Category: Lysis Cassette, parts | ||
|- | |- | ||
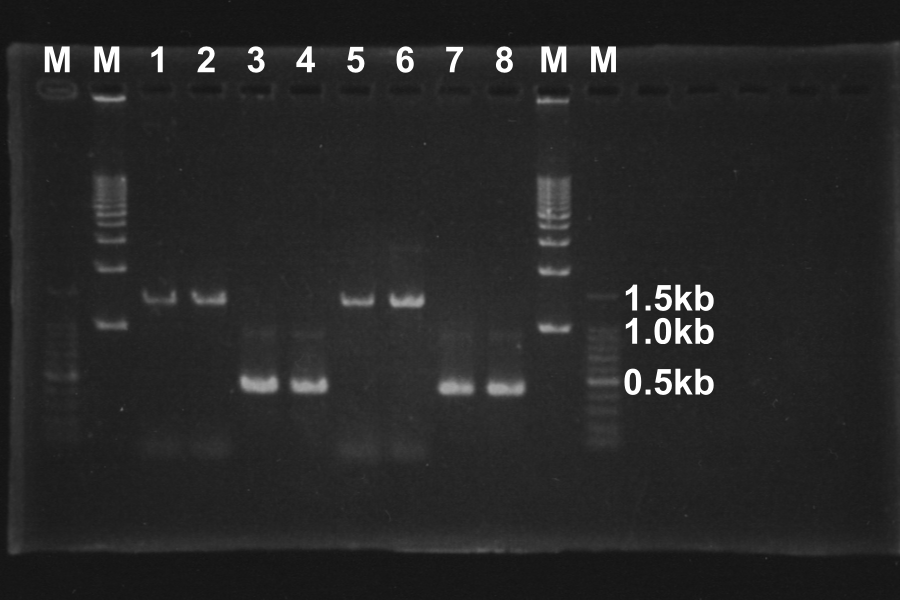
| - | |1 | + | |(1) Electrophoresis of the PCR products for 40min. |
[[Image:KyotoExp100722-1.png|right]] | [[Image:KyotoExp100722-1.png|right]] | ||
* '''Discussion''' | * '''Discussion''' | ||
* Length of S and S-R-Rz/Rz1 is 370bp, 1300bp, so PCR succeeded. | * Length of S and S-R-Rz/Rz1 is 370bp, 1300bp, so PCR succeeded. | ||
|- | |- | ||
| - | |2 | + | |(2) [[Team:Kyoto/Protocols#Miniprep|Miniprep]] of iGEM Parts. |
{| class="experiments" | {| class="experiments" | ||
!Name||Concentration(ng/µl) | !Name||Concentration(ng/µl) | ||
| Line 129: | Line 129: | ||
* The concentration of all samples was very week. Probably our shaking incubation was week. | * The concentration of all samples was very week. Probably our shaking incubation was week. | ||
|- | |- | ||
| - | |3 | + | |(3) Culture plates and make master plates of <partinfo>pSB4K5</partinfo> and <partinfo>B0015</partinfo> from 7/22 17:00 to 7/23 10:00. |
|} | |} | ||
---- | ---- | ||
| Line 140: | Line 140: | ||
* Category: | * Category: | ||
|- | |- | ||
| - | |1 | + | |(1) [[Team:Kyoto/Protocols#Miniprep|Miniprep]] of iGEM Parts. |
{| class="experiments" | {| class="experiments" | ||
!Name||Concentration(ng/µl) | !Name||Concentration(ng/µl) | ||
| Line 152: | Line 152: | ||
* The concentration of <partinfo>pSB4K5</partinfo> is high, so this condition of shaking incubation is moderate. | * The concentration of <partinfo>pSB4K5</partinfo> is high, so this condition of shaking incubation is moderate. | ||
|- | |- | ||
| - | |2 | + | |(2) Picked up 1, 3, 5, 7 of the products of PCR, and purified by PCR-purification. |
{| class="experiments" | {| class="experiments" | ||
!Sample||Concentration (ng/µl)||New Name|| | !Sample||Concentration (ng/µl)||New Name|| | ||
| Line 167: | Line 167: | ||
* The concentration of sample number 1 and 5, the PCR products of S-R-Rz, is week, so we desided to retry PCR. | * The concentration of sample number 1 and 5, the PCR products of S-R-Rz, is week, so we desided to retry PCR. | ||
|- | |- | ||
| - | |3 | + | |(3) Retry of PCR of S-R-Rz/Rz1. |
{| class="experiments" | {| class="experiments" | ||
!Sample||Water||25mM MgSO4||2mM dNTPs||10×Buffer for KOD plus ver.2||Template DNA (5ng/µl)||Primer S-R-Rz/Rz1 Forward (10µM)||Primer S-R-Rz/Rz1 Reverse (10µM)||KOD plus ver.2||Total | !Sample||Water||25mM MgSO4||2mM dNTPs||10×Buffer for KOD plus ver.2||Template DNA (5ng/µl)||Primer S-R-Rz/Rz1 Forward (10µM)||Primer S-R-Rz/Rz1 Reverse (10µM)||KOD plus ver.2||Total | ||
| Line 185: | Line 185: | ||
* PCR condition : 94℃ x 2min, (98℃ x 10sec, 55℃ x 30sec, 68℃ x 1min) x 30cycles, 4℃ forever. | * PCR condition : 94℃ x 2min, (98℃ x 10sec, 55℃ x 30sec, 68℃ x 1min) x 30cycles, 4℃ forever. | ||
|- | |- | ||
| - | |4 | + | |(4) Digested <partinfo>J06702</partinfo> by EcoRI, XbaI, SpeI, PstI to check function of our Restriction enzymes. |
{| class="experiments" | {| class="experiments" | ||
!Sample||10xBuffer||BSA||Enzyme||MilliQ||Total||Incubation | !Sample||10xBuffer||BSA||Enzyme||MilliQ||Total||Incubation | ||
| Line 200: | Line 200: | ||
|} | |} | ||
|- | |- | ||
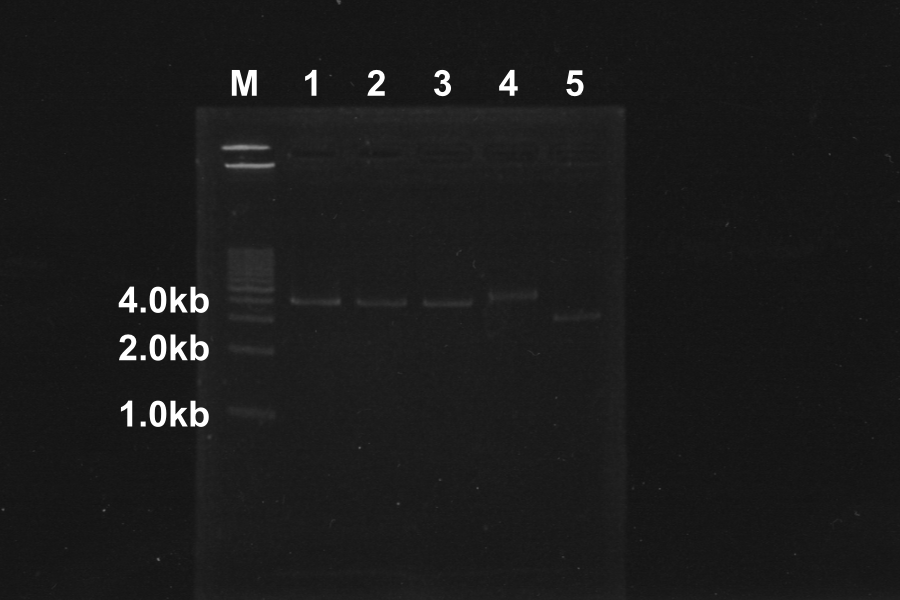
| - | |5 | + | |(5) Electrophoresis of above sample for 35min. |
[[Image:KyotoExp100723-1.png|right]] | [[Image:KyotoExp100723-1.png|right]] | ||
* '''Discussion''' | * '''Discussion''' | ||
* Comparison to sample 5(control, circular DNA), the bands of sample 1, 2, 3, 4 was shifted. The DNA of sample 1, 2, 3, 4 was linearized by Restriction enzymes. So, our restriction enzymes work correctly. | * Comparison to sample 5(control, circular DNA), the bands of sample 1, 2, 3, 4 was shifted. The DNA of sample 1, 2, 3, 4 was linearized by Restriction enzymes. So, our restriction enzymes work correctly. | ||
|- | |- | ||
| - | |6 | + | |(6) To insert S gene to GFP, we digested the PCR products of S gene by EcoRi and SpeI, and GFP by EcoRl and XbaI. |
{| class="experiments" | {| class="experiments" | ||
!Sample||10×Buffer||Enzyme 1||Enzyme 2||MilliQ||Total||Incubation | !Sample||10×Buffer||Enzyme 1||Enzyme 2||MilliQ||Total||Incubation | ||
Revision as of 16:27, 27 August 2010
Contents |
Index
Notebook
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(1) Solubilized of antibiotics, Ampicillin and Kanamycin as the following.
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| (2) Made plates for LB (Ampicillin+) and LB (Kanamycin+). | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(3) Transformed iGEM Parts.
|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| (1) Cultured plates in which colonies was observed at 37℃ from 07/21 20:50 to 07/22 17:00. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| (2) Made a master plate of the above plates. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(3) Retried Transformation of iGEM Parts.
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(4) PCR for S-R-Rz/Rz1 and S
|
| ||||||||||||||
(1) Electrophoresis of the PCR products for 40min.
| ||||||||||||||
(2) Miniprep of iGEM Parts.
| ||||||||||||||
| (3) Culture plates and make master plates of <partinfo>pSB4K5</partinfo> and <partinfo>B0015</partinfo> from 7/22 17:00 to 7/23 10:00. |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(1) Miniprep of iGEM Parts.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(2) Picked up 1, 3, 5, 7 of the products of PCR, and purified by PCR-purification.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(3) Retry of PCR of S-R-Rz/Rz1.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(4) Digested <partinfo>J06702</partinfo> by EcoRI, XbaI, SpeI, PstI to check function of our Restriction enzymes.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(5) Electrophoresis of above sample for 35min.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(6) To insert S gene to GFP, we digested the PCR products of S gene by EcoRi and SpeI, and GFP by EcoRl and XbaI.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Ligated over night
|
 "
"