Team:KAIST-Korea/Project/Methods
From 2010.igem.org
(→Cloning) |
|||
| Line 4: | Line 4: | ||
<tr> | <tr> | ||
<td valign="top" width="75%"> | <td valign="top" width="75%"> | ||
| - | == Cloning == | + | == Cloning Plan == |
| - | + | ||
<br> | <br> | ||
| - | Aim | + | Aim |
<br> | <br> | ||
# Build DiscoverY yeast: transform genes to S.pombe and turn it into the yeast we want. | # Build DiscoverY yeast: transform genes to S.pombe and turn it into the yeast we want. | ||
| Line 31: | Line 30: | ||
Insert RE treated gene to TA cloning vector -> Ligation -> E.coli transformation -> Prep -> Loading (2 d) – team | Insert RE treated gene to TA cloning vector -> Ligation -> E.coli transformation -> Prep -> Loading (2 d) – team | ||
| + | |||
== PCR == | == PCR == | ||
Revision as of 12:47, 23 August 2010
Cloning Plan
PCR To manipulate gene products obtained from gene-bank, our team used PCR/Real-Time PCR methods. Commercially obtained genes contain few more base pairs added at front and back site. As for FGPR, the receptor protein of human has human-specific signal peptide at its starting point, and this region had to be replaced by S.pombe specific signal peptide to express the protein at membrane region. To do this, PCR primers containing signal peptide region of S.pombe were synthesized by method describeds below. Then, FGPR gene was amplified by PCR to get gene product whose signal region is replaced. Also, additional sequence at rear site of gene was removed. STAT gene went through similar process; removing additional region. Oligo Synthesis Oligo-synthesis was supported by Bioneer. Codon optimization Oligo Design S/W
Mega-base Oligo Synthesizer
AccuRapid Cell-Free Protein Expression Kit
Gene Synthesis Kit
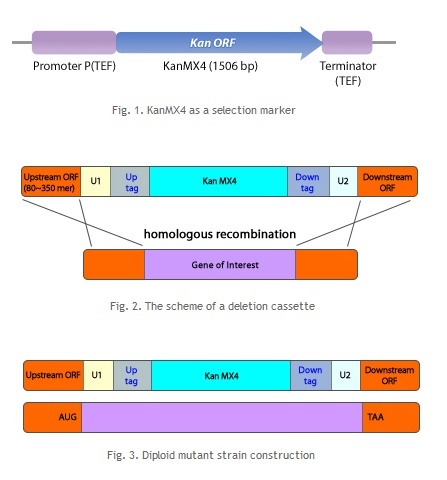
Homologous Recombination Homologous recombination is a type of genetic recombination in which nucleotide sequences are exchanged between two similar or identical molecules of DNA. It is most widely used by cells to accurately repair harmful breaks that occur on both strands of DNA, known as double-strand breaks. Homologous recombination also produces new combinations of DNA sequences during meiosis, the process performed by many eukaryotes like animals and plants: production of sperm and egg cells. These new combinations of DNA promote genetic variations in offspring, which in turn enable populations to evolve. Homologous recombination is also used in horizontal gene transfer to exchange genetic material between different strains and species of bacteria and viruses.
ReferencesBioneer PCR service: |
 "
"