Team:Stockholm/4 August 2010
From 2010.igem.org
(→Andreas) |
|||
| Line 39: | Line 39: | ||
===Fermentas/ThermoFisher Scientific sponsorship=== | ===Fermentas/ThermoFisher Scientific sponsorship=== | ||
Called Fermentas/ThermoFisher Scientific and got a small (but much appreciated) sponsorship for FastDigest AgeI restriction enzymes. Their logotype was added to our dedicated sponsorship page. | Called Fermentas/ThermoFisher Scientific and got a small (but much appreciated) sponsorship for FastDigest AgeI restriction enzymes. Their logotype was added to our dedicated sponsorship page. | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | == Mimmi== | ||
| + | |||
| + | |||
| + | === MITF === | ||
| + | ==== - Gel ==== | ||
| + | |||
| + | |||
| + | *0.5% agarose gel | ||
| + | *No products! | ||
| + | :--> Primers are double and triple checked... Trying a gradient! | ||
| + | |||
| + | |||
| + | |||
| + | ==== - amplifying and moving ==== | ||
| + | |||
| + | |||
| + | *the PCR products (from 2010-08-02) are treated with the restriction enzyme AgeI | ||
| + | **the mutated MITF should show two bands: ~1300bp and ~10bp (which you dont see) | ||
| + | **the control (non-mutated) MITF should show three bands: ~1060bp, ~200bp and ~10bp (which you don't see) | ||
| + | |||
| + | |||
| + | |||
| + | {| | ||
| + | ! dNTP 10µM | ||
| + | | (µl) | ||
| + | |- | ||
| + | | ATP 100µM | ||
| + | | 7 | ||
| + | |- | ||
| + | | CTP 100µM | ||
| + | | 7 | ||
| + | |- | ||
| + | | GTP 100µM | ||
| + | | 7 | ||
| + | |- | ||
| + | | TTP 100µM | ||
| + | | 7 | ||
| + | |- | ||
| + | | sH<sub>2</sub>O | ||
| + | | 52 | ||
| + | | align="right" | tot | ||
| + | | 70 | ||
| + | |} | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | {| | ||
| + | ! Mix | ||
| + | | (µl) | ||
| + | | X18 | ||
| + | | rowspan="10" width="150" | | ||
| + | !colspan="2" | conditions | ||
| + | | rowspan="3" width="150" | | ||
| + | |- | ||
| + | | sH<sub>2</sub>O | ||
| + | | 39.5 | ||
| + | | 711 | ||
| + | ! time | ||
| + | ! °C | ||
| + | |- | ||
| + | | F primer | ||
| + | | 0.75 | ||
| + | | 13.5 | ||
| + | | 2m | ||
| + | | 94 | ||
| + | |- | ||
| + | | R primer | ||
| + | | 0.75 | ||
| + | | 13.5 | ||
| + | | 30s | ||
| + | | 94 | ||
| + | | ) | ||
| + | |- | ||
| + | | buffer | ||
| + | | 5 | ||
| + | | 90 | ||
| + | | 30s | ||
| + | | 45, 50, 55 | ||
| + | | > 5 cycles | ||
| + | |- | ||
| + | | dNTPs 10µM | ||
| + | | 1.5 | ||
| + | | 27 | ||
| + | | 1m30s | ||
| + | | 68 | ||
| + | | ) | ||
| + | |- | ||
| + | | MgSO<sub>4</sub> 50µM | ||
| + | | 1 | ||
| + | | 18 | ||
| + | | 30s | ||
| + | | 94 | ||
| + | | \ | ||
| + | |- | ||
| + | | polymerase | ||
| + | | 0.5 | ||
| + | | 6 | ||
| + | | 1m30s | ||
| + | | 68 | ||
| + | | / 25 cycles | ||
| + | |- | ||
| + | | DNA | ||
| + | | 1 | ||
| + | | 18X1 | ||
| + | | 10m | ||
| + | | 68 | ||
| + | | rowspan="2" | | ||
| + | |- | ||
| + | | align="right" | tot | ||
| + | | 50 | ||
| + | | | ||
| + | | oo | ||
| + | | 10 | ||
| + | |} | ||
Revision as of 20:10, 10 August 2010
Contents |
Andreas
Cloning results
From 3/8 transformations
| pSB1A3.SOD: | Bacterial lawn on all three plates, probably due to the Amp in plates having degraded. Plates discarded |
| pSB1A3.yCCS A: | |
| pSB1A3.yCCS B: | |
| pSB1C3.IgG prot.: | Good colony yield. Left to grow and mature for 4-5 more hours until red colonies appeared. |
ON cultures
Four colonies picked from IgG protease plate and resuspended in 10 μl LB. 3 μl used to inoculate 5 ml LB + 25 Cm. Grown ON in 37 °C, 250 rpm.
Cloning/transformations
New quick-transformations were performed for:
- pSB1A3.SOD
- pSB1A3.yCCS A
- pSB1A3.yCCS B
Procedures as described 3/8.
CPP DNA synthesis
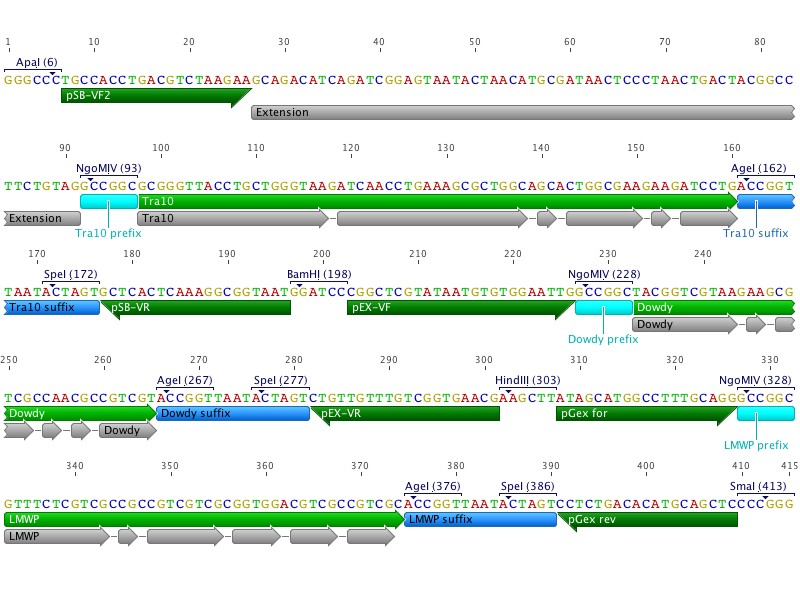
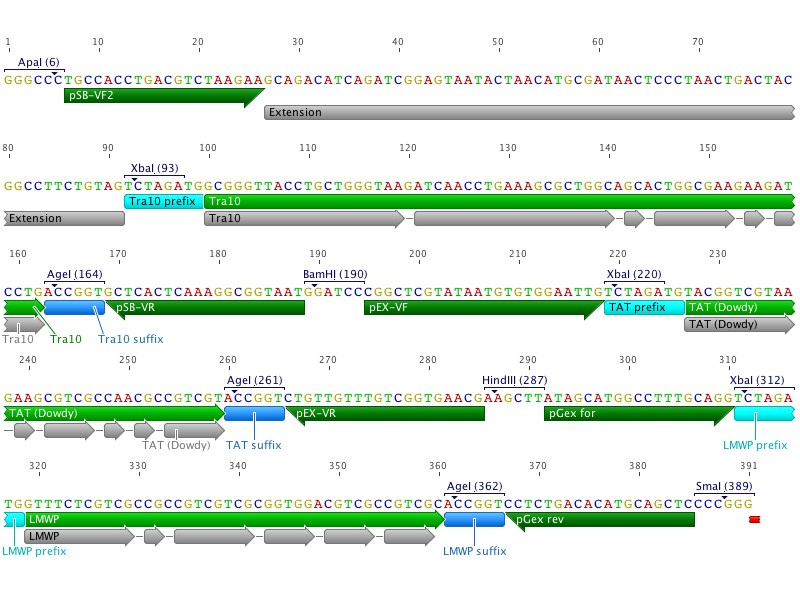
Since our previous CPP synthesis order was rejected due to repetetive sequences Johan and I redesigned the order into two separate clusters, CPP_N and CPP_C. The new clusters were designed with both restriction sites and primer annealing sites flanking each CPP. An extension sequence was also added to enable gel separation after digestion, as the CPPs are very similar in length.
Johan also redesigned the coding sequence by shifting codons by the addition of "silent mutations".
Fermentas/ThermoFisher Scientific sponsorship
Called Fermentas/ThermoFisher Scientific and got a small (but much appreciated) sponsorship for FastDigest AgeI restriction enzymes. Their logotype was added to our dedicated sponsorship page.
Mimmi
MITF
- Gel
- 0.5% agarose gel
- No products!
- --> Primers are double and triple checked... Trying a gradient!
- amplifying and moving
- the PCR products (from 2010-08-02) are treated with the restriction enzyme AgeI
- the mutated MITF should show two bands: ~1300bp and ~10bp (which you dont see)
- the control (non-mutated) MITF should show three bands: ~1060bp, ~200bp and ~10bp (which you don't see)
| dNTP 10µM | (µl) | ||
|---|---|---|---|
| ATP 100µM | 7 | ||
| CTP 100µM | 7 | ||
| GTP 100µM | 7 | ||
| TTP 100µM | 7 | ||
| sH2O | 52 | tot | 70 |
| Mix | (µl) | X18 | conditions | |||
|---|---|---|---|---|---|---|
| sH2O | 39.5 | 711 | time | °C | ||
| F primer | 0.75 | 13.5 | 2m | 94 | ||
| R primer | 0.75 | 13.5 | 30s | 94 | ) | |
| buffer | 5 | 90 | 30s | 45, 50, 55 | > 5 cycles | |
| dNTPs 10µM | 1.5 | 27 | 1m30s | 68 | ) | |
| MgSO4 50µM | 1 | 18 | 30s | 94 | \ | |
| polymerase | 0.5 | 6 | 1m30s | 68 | / 25 cycles | |
| DNA | 1 | 18X1 | 10m | 68 | ||
| tot | 50 | oo | 10 | |||
 "
"