Team:KAIST-Korea/Notebook/Memo/Etc
From 2010.igem.org
Yangstefano (Talk | contribs) (→Etc. We handle information and idea at ETC) |
Yangstefano (Talk | contribs) |
||
| Line 79: | Line 79: | ||
8. 'Y.canD' expresse GFP.</td></tr> | 8. 'Y.canD' expresse GFP.</td></tr> | ||
</table> | </table> | ||
| + | </td></tr></table> | ||
| + | <br><br> | ||
| + | <span style=font-size:15px> <b> Why Yeast? </b> </span> | ||
| + | <br> | ||
| + | Why we use ‘Yeast’ instead of ‘E. coli’? | ||
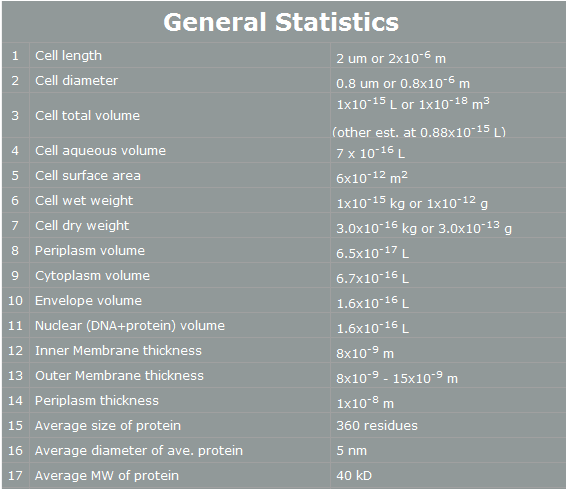
| + | E. coli control is easier than Yeast control. E. coli has faster cell cycle than Yeast. Moreover, there are many E. coli templates. But, although E. coli has many profits, we choose Yeast. Why? The following table is E. coli properties.[2] | ||
| + | <br><br> | ||
| + | [[Image:E.coli general statistics.jpg|500px|center]] | ||
| + | <br> | ||
| + | According to the table, we can find easily some reasons that E. coli cannot work human pathway well.<br><br> | ||
| + | First, the periplasm’s thickness of E. coli is the very small as 10nm. In our project, we have to use human proteins. Almost human proteins are much bigger than E. coli’s proteins. (They are over 10nm*10nm*10nm.) Furthermore, in our project, STAT3 has 60nm height. Therefore, this protein cannot be in periplasm, and our pathway cannot work completely.[3] <br><br> | ||
| + | Second, the average E. coli protein has only 360 residues, 5nm diameter, and 40kD MW. As we mentioned, our project’s proteins have over 1000residues, 10nm diameter and 50kD MW. Thus, the possibility that E. coli have our pathway is very low. <br><br> | ||
| + | However, Yeast has big volume size from 3~4 µm to 40 µm. [4] And Yeast is a eukaryotic, so it is more similar to human cell than E. coli. There is no physical problem of using human protein in yeast. And has more possibility to do our pathway. Therefore, we use Yeast instead of E. coli. | ||
| + | <br><br> | ||
| + | <span style=font-size:15px> <b> Biochemical Receptors </b> </span> | ||
| + | <br> | ||
| + | [[Image:Int5.png|Biochemical Receptors|center]] | ||
| + | <br> | ||
| + | The upper table represents classification of all biological receptors. First of all, since our genetically engineered machine has to detect on mycobacteria directly, we should select cell surface receptors. Next, we should consider using fusion-antibody-receptors. We used gene combination simply (add antibody to biological receptor without original receipt part). If the receptor has conformational change property when it accepts input signal, we guess that our fusion antibody receptor cannot operate well because antibodies characteristics (antigen size, antibody size, etc.) are different. They don’t have special receipt part in a whole receptor, of course, so whole receptor is important receipt part; we cannot create fusion-antibody-receptor with this protein anyway. Therefore, we choose ‘Dimerization’ receptor. Most of these receptors have Immunoglobulin-like receptor part which can be alternated by antibodies easily. Also, since biosensors should activate gene expression, we selected gene-regulation related receptors. <br><br> | ||
| + | Finally we choose cytokine receptors and receptor tyrosine kinases. Among them, interleukin-6 alpha receptor was selected. | ||
| + | <br><br> | ||
| + | <span style=font-size:15px> <b> Fusion Antibody Receptors </b> </span> | ||
| + | <br> | ||
| + | '''The Fusion Antibody-Receptor''' is a protein which receptor genetically combines with a specific antibody. The following figures show what the fusion antibody-receptor is for details. | ||
| + | <br><br> | ||
| + | [[Image:Int7.png|Fusion Antibody Receptors|center|620px]] | ||
| + | <br> | ||
| + | The fusion antibody-receptor is designed by simple gene modification. We altered the ''Mycobacterium tuberculosis'' antigen detectable antibody single chain instead of the cytokine receptor’s antigen acceptable part (Immunoglobulin-like parts). Light chain part of the antibody – Linker – Heavy chain part of antibody is enough. (There are some evidences to success making fusion antibody-receptor with this method in some experiments. [4]) Then in order to express the receptor on cell surface membrane of Yeast, we change the original signal peptide to Omp(Outer membrane protein) signal peptide of Yeast. Surely the receptor should satisfy our standard for biosensor. | ||
| + | <br> | ||
| + | [[Image:Int8.png|Signal Peptide|center|620px]] | ||
| + | <br> | ||
| + | <b>Schizosaccharomyces pombe Signal peptide</b> from Cell wall integrity and stress response component 1 <br> | ||
| + | Protein sequence: <br> | ||
| + | MVFLNSSPFKGRLLFFVYLLIISTRLVAA <br> | ||
| - | </ | + | mRNA sequece:<br> |
| + | atggtgtttctgaacagcagcccgtttaaaggccgcctgctgttttttgtgtatctgctgattattagcacccgcctggtggcggcg <br> | ||
| + | <br><br> | ||
| + | <span style=font-size:15px> <b> Signal Pathway : JAK-STAT Pathway </b> </span> | ||
| + | <br> | ||
| + | There are many signal pathways from plasma membrane receptor to the gene regulation. Figure B is an example of these pathways, MAP kinase pathway linked with RAS pathway. In this pathway, | ||
| + | |||
| + | # With existence of signal molecule, receptor kinases dimerize themselves and phosphorylate each other. | ||
| + | # This phosphorylated receptor dimer is cognized by Grb2 and recruit SOS protein, the Guanine nucleotide Exchange Factor(GEP) | ||
| + | # Recruited SOS protein substitute as GDP of Ras protein with GTP. | ||
| + | # Ras protein with GTP changes its conformation and activate its own kinase activity(Ras pathway so far) | ||
| + | # Ras kinase activate the MAP kinase kinase kinase, MAP KKK(also called as Raf protein) with phosphorylation | ||
| + | # MAP KKK activates the MAP kinase kinase, MAP KK(also called as Mek) with phosphorylation | ||
| + | # MAP KK activates the MAP kinase, MAP K(also called as Erk) with phosphorylation | ||
| + | # Activated MAP Kinase phosphorylates many cytosol proteins. | ||
| + | # For example, if Jun TF is phosphorylated by MAP K, phosphorylated Jun go inside of nucleus and activate the transcription to bind to DNA. (MAP pathway so far) | ||
| + | |||
| + | |||
| + | But to port this signal pathway into the Yeast is not easy because this pathway is related with too many new proteins, while JAK-STAT pathway is easy to port because it doesn’t have many participating proteins. The signal transduction step of JAK-STAT pathway as follows. | ||
| + | <br><br> | ||
| + | [[Image:pathway1.jpg|620px|center]] | ||
| + | <br><br> | ||
| + | # Cytokine binds to the cytokine receptor with JAK kinase. | ||
| + | # Kinases form dimer and are phosphorylated by JAK. | ||
| + | # These phosphorylated tyrosine residue is cognized by STAT protein. | ||
| + | # STAT which cognize phosphorylated tyrosine phosphorylate other STAT protein. | ||
| + | # Two phosphorylated STAT protein form dimer and go inside to the nucleus and activate near gene to bind to the APRE element. | ||
| + | |||
| + | Because JAK-STAT pathway have only 5 steps while RAS-MAP pathway require 9 steps, we decide to port JAK-STAT pathway to the Yeast. | ||
| + | <br><br> | ||
| + | [[Image:pathway2.jpg|620px|center]]<br><br> | ||
| + | Actually, there are many JAK-STAT pathways in human cell. JAK and STAT are important components of many cytokine receptor systems. According to combination of the receptor, JAK and STAT, there exits many pathways which acts different works in eukaryotic cell. Among these pathways, we choose IL-6α, JAK1, STAT3 pathway. In this pathway, gp130 dimer and LMO4 are combined with JAK1, and assists a whole response. Why we selected the IL-6α specially? That is, IL-6α is a unique receptor which has a Ig-like region at outer part as different with other cytokine receptors. The introduction of IL-6α, gp130, and LMO4 are below. | ||
| + | <br><br> | ||
| + | [[Image:Int6.png|Interleukin Receptors|center|600px]] | ||
| + | <br><br> | ||
| + | [[Image:gp130 LMO4.jpg|620px|center]] | ||
| + | <br><br> | ||
| + | <span style=font-size:15px> <b> Gene Activation for Output </b> </span> | ||
| + | <br> | ||
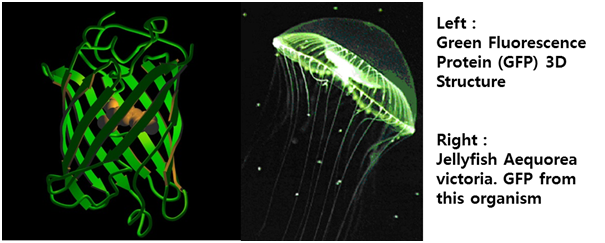
| + | STAT1 and STAT3 proteins with the formation of STAT3/3 and STAT1/3 dimers are confirmed using probe, namely the APRE probe. '''APRE probe'''(''A''cute-''p''hase ''r''esponse ''e''lement probe) is the acute phase responsive element of the α2M gene promoter and known to bind STAT1 and STAT3 proteins. Using this pathway, we altered the '''green fluorescent protein (GFP) gene''' instead of cytokine inducible genes. So, we could show GFP expression through the previous whole pathway.'''[Fig. 5]''' | ||
| + | <br><br> | ||
| + | [[Image:Int9.png|Figure. 5|center]] | ||
| + | <br><br> | ||
Revision as of 08:38, 16 July 2010
Etc. We handle information and idea at ETC
|
Why Yeast?
Why we use ‘Yeast’ instead of ‘E. coli’?
E. coli control is easier than Yeast control. E. coli has faster cell cycle than Yeast. Moreover, there are many E. coli templates. But, although E. coli has many profits, we choose Yeast. Why? The following table is E. coli properties.[2]
According to the table, we can find easily some reasons that E. coli cannot work human pathway well.
First, the periplasm’s thickness of E. coli is the very small as 10nm. In our project, we have to use human proteins. Almost human proteins are much bigger than E. coli’s proteins. (They are over 10nm*10nm*10nm.) Furthermore, in our project, STAT3 has 60nm height. Therefore, this protein cannot be in periplasm, and our pathway cannot work completely.[3]
Second, the average E. coli protein has only 360 residues, 5nm diameter, and 40kD MW. As we mentioned, our project’s proteins have over 1000residues, 10nm diameter and 50kD MW. Thus, the possibility that E. coli have our pathway is very low.
However, Yeast has big volume size from 3~4 µm to 40 µm. [4] And Yeast is a eukaryotic, so it is more similar to human cell than E. coli. There is no physical problem of using human protein in yeast. And has more possibility to do our pathway. Therefore, we use Yeast instead of E. coli.
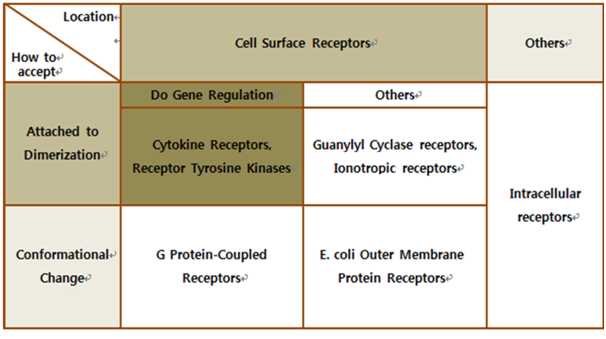
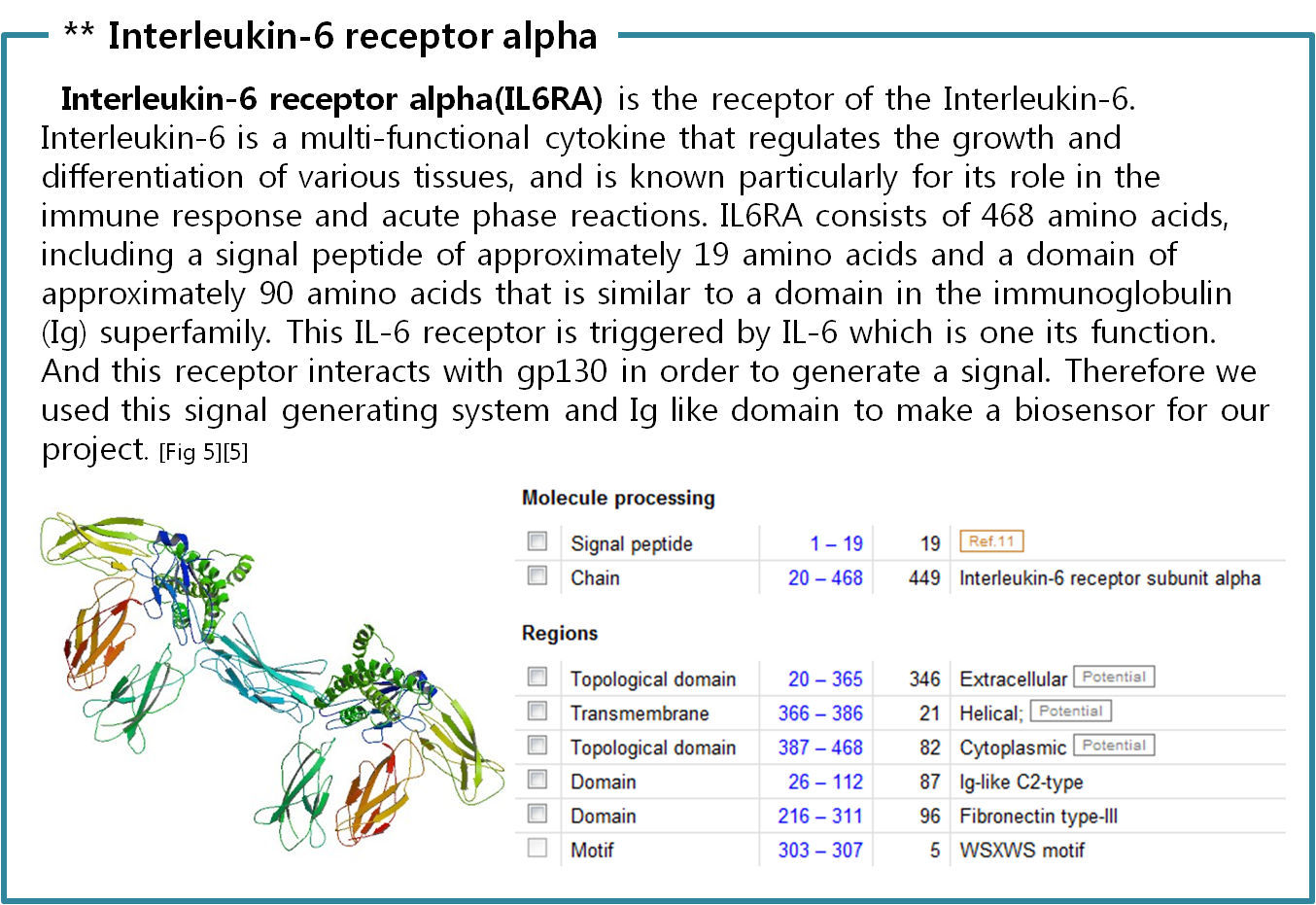
Biochemical Receptors
The upper table represents classification of all biological receptors. First of all, since our genetically engineered machine has to detect on mycobacteria directly, we should select cell surface receptors. Next, we should consider using fusion-antibody-receptors. We used gene combination simply (add antibody to biological receptor without original receipt part). If the receptor has conformational change property when it accepts input signal, we guess that our fusion antibody receptor cannot operate well because antibodies characteristics (antigen size, antibody size, etc.) are different. They don’t have special receipt part in a whole receptor, of course, so whole receptor is important receipt part; we cannot create fusion-antibody-receptor with this protein anyway. Therefore, we choose ‘Dimerization’ receptor. Most of these receptors have Immunoglobulin-like receptor part which can be alternated by antibodies easily. Also, since biosensors should activate gene expression, we selected gene-regulation related receptors.
Finally we choose cytokine receptors and receptor tyrosine kinases. Among them, interleukin-6 alpha receptor was selected.
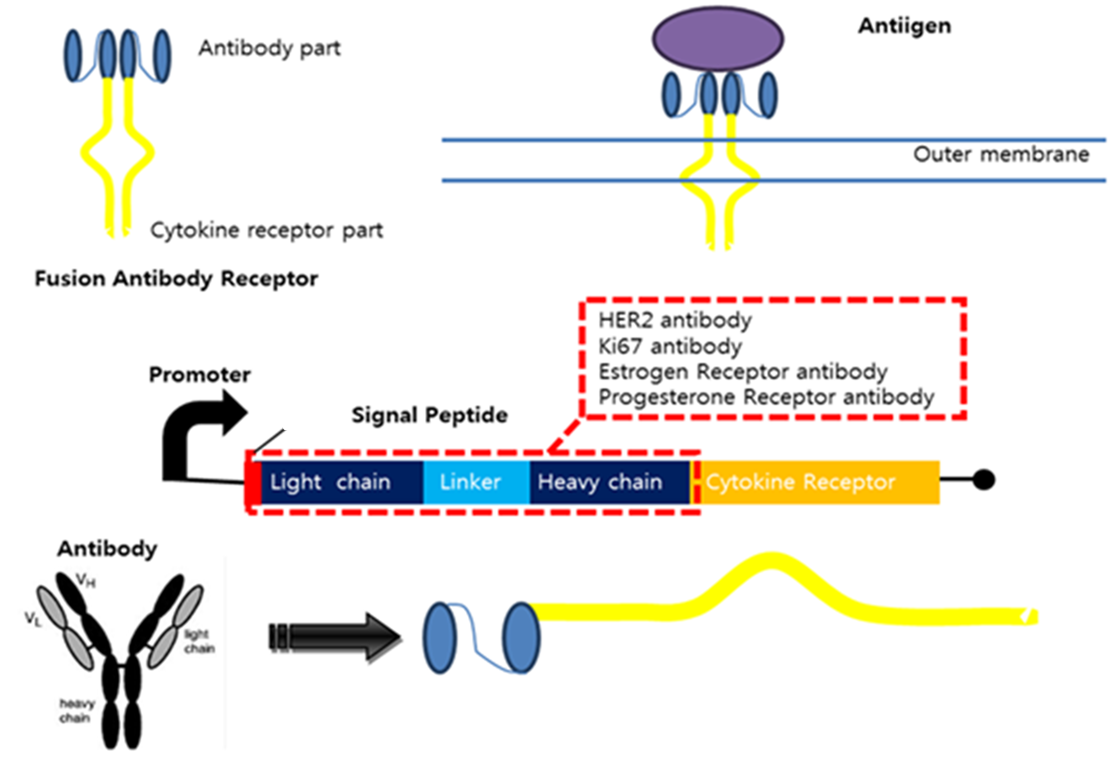
Fusion Antibody Receptors
The Fusion Antibody-Receptor is a protein which receptor genetically combines with a specific antibody. The following figures show what the fusion antibody-receptor is for details.
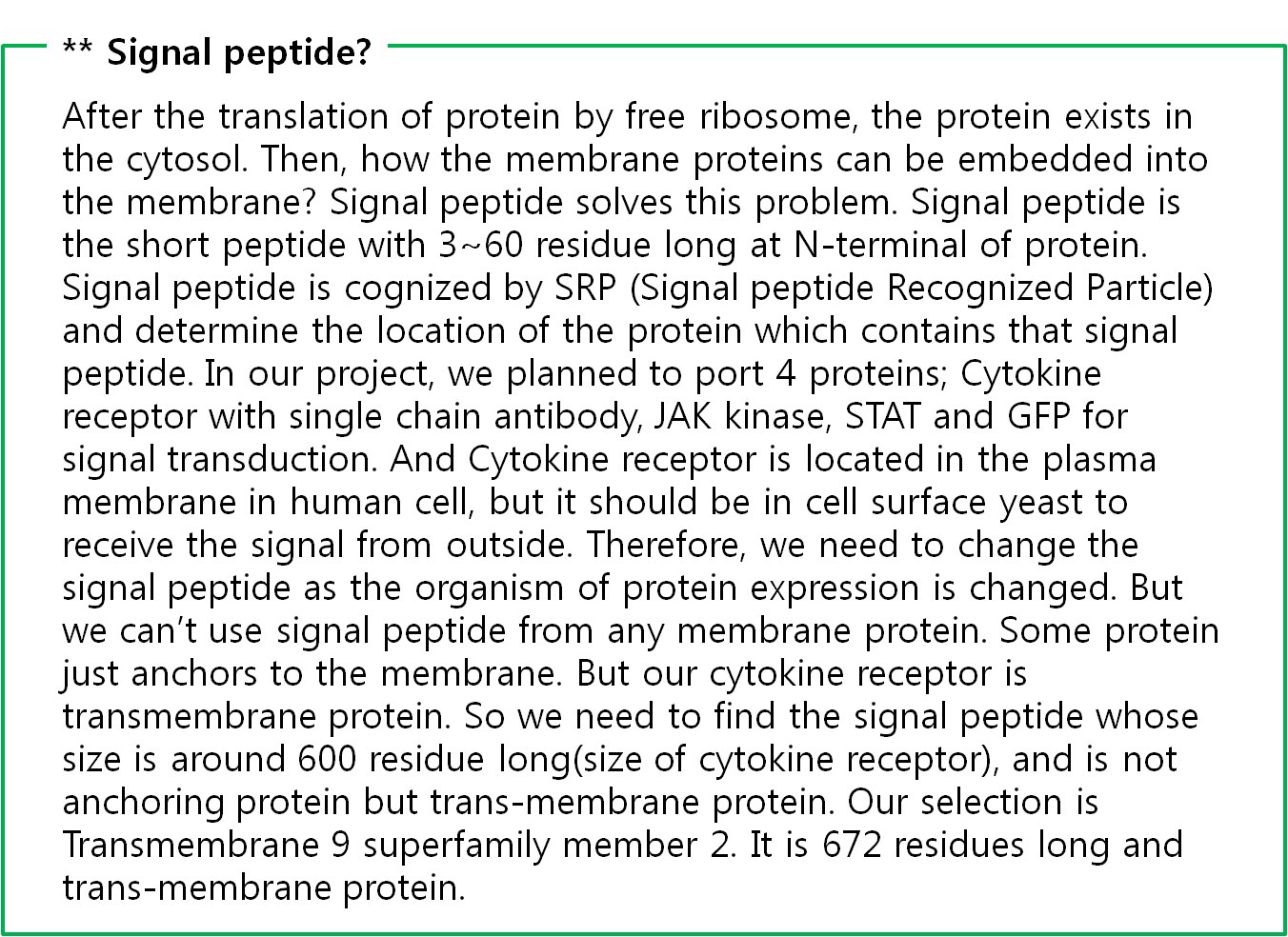
The fusion antibody-receptor is designed by simple gene modification. We altered the Mycobacterium tuberculosis antigen detectable antibody single chain instead of the cytokine receptor’s antigen acceptable part (Immunoglobulin-like parts). Light chain part of the antibody – Linker – Heavy chain part of antibody is enough. (There are some evidences to success making fusion antibody-receptor with this method in some experiments. [4]) Then in order to express the receptor on cell surface membrane of Yeast, we change the original signal peptide to Omp(Outer membrane protein) signal peptide of Yeast. Surely the receptor should satisfy our standard for biosensor.
Schizosaccharomyces pombe Signal peptide from Cell wall integrity and stress response component 1
Protein sequence:
MVFLNSSPFKGRLLFFVYLLIISTRLVAA
mRNA sequece:
atggtgtttctgaacagcagcccgtttaaaggccgcctgctgttttttgtgtatctgctgattattagcacccgcctggtggcggcg
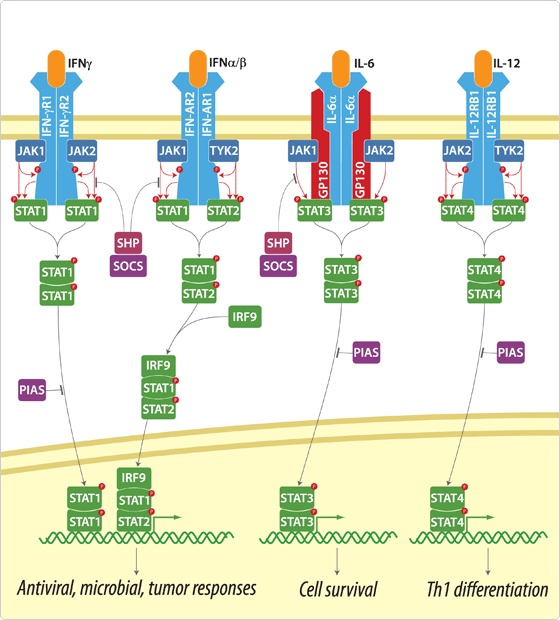
Signal Pathway : JAK-STAT Pathway
There are many signal pathways from plasma membrane receptor to the gene regulation. Figure B is an example of these pathways, MAP kinase pathway linked with RAS pathway. In this pathway,
- With existence of signal molecule, receptor kinases dimerize themselves and phosphorylate each other.
- This phosphorylated receptor dimer is cognized by Grb2 and recruit SOS protein, the Guanine nucleotide Exchange Factor(GEP)
- Recruited SOS protein substitute as GDP of Ras protein with GTP.
- Ras protein with GTP changes its conformation and activate its own kinase activity(Ras pathway so far)
- Ras kinase activate the MAP kinase kinase kinase, MAP KKK(also called as Raf protein) with phosphorylation
- MAP KKK activates the MAP kinase kinase, MAP KK(also called as Mek) with phosphorylation
- MAP KK activates the MAP kinase, MAP K(also called as Erk) with phosphorylation
- Activated MAP Kinase phosphorylates many cytosol proteins.
- For example, if Jun TF is phosphorylated by MAP K, phosphorylated Jun go inside of nucleus and activate the transcription to bind to DNA. (MAP pathway so far)
But to port this signal pathway into the Yeast is not easy because this pathway is related with too many new proteins, while JAK-STAT pathway is easy to port because it doesn’t have many participating proteins. The signal transduction step of JAK-STAT pathway as follows.
- Cytokine binds to the cytokine receptor with JAK kinase.
- Kinases form dimer and are phosphorylated by JAK.
- These phosphorylated tyrosine residue is cognized by STAT protein.
- STAT which cognize phosphorylated tyrosine phosphorylate other STAT protein.
- Two phosphorylated STAT protein form dimer and go inside to the nucleus and activate near gene to bind to the APRE element.
Because JAK-STAT pathway have only 5 steps while RAS-MAP pathway require 9 steps, we decide to port JAK-STAT pathway to the Yeast.
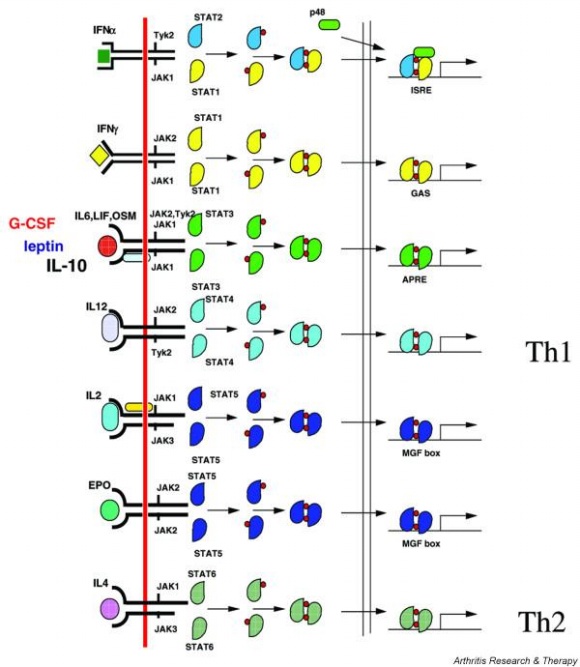
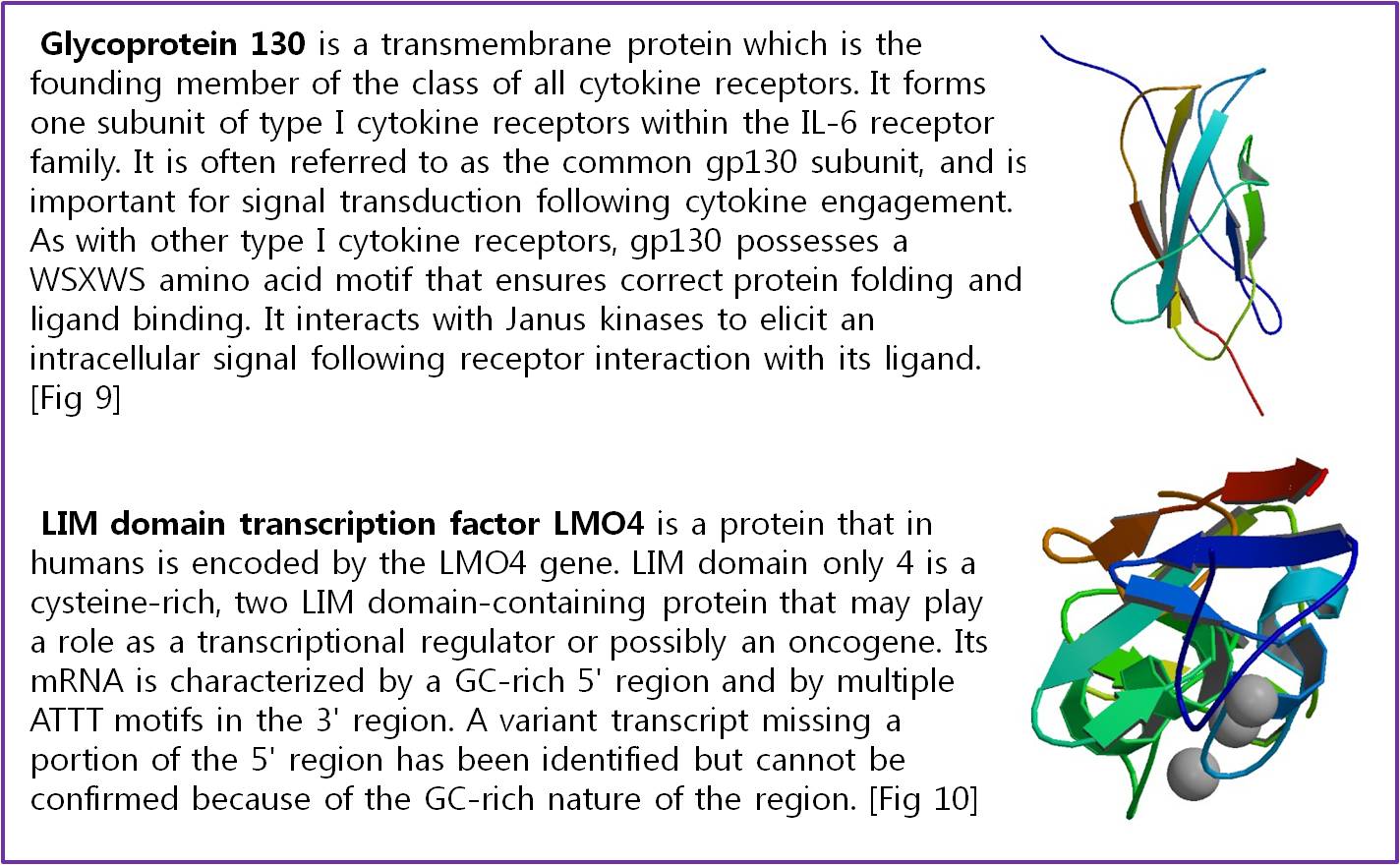
Actually, there are many JAK-STAT pathways in human cell. JAK and STAT are important components of many cytokine receptor systems. According to combination of the receptor, JAK and STAT, there exits many pathways which acts different works in eukaryotic cell. Among these pathways, we choose IL-6α, JAK1, STAT3 pathway. In this pathway, gp130 dimer and LMO4 are combined with JAK1, and assists a whole response. Why we selected the IL-6α specially? That is, IL-6α is a unique receptor which has a Ig-like region at outer part as different with other cytokine receptors. The introduction of IL-6α, gp130, and LMO4 are below.
Gene Activation for Output
STAT1 and STAT3 proteins with the formation of STAT3/3 and STAT1/3 dimers are confirmed using probe, namely the APRE probe. APRE probe(Acute-phase response element probe) is the acute phase responsive element of the α2M gene promoter and known to bind STAT1 and STAT3 proteins. Using this pathway, we altered the green fluorescent protein (GFP) gene instead of cytokine inducible genes. So, we could show GFP expression through the previous whole pathway.[Fig. 5]
 "
"