Team:Cambridge/Bioluminescence/Luciferin Regeneration
From 2010.igem.org
| Line 8: | Line 8: | ||
[http://www.ncbi.nlm.nih.gov/pubmed/11457857 Gomi and Kajiyama (2001)] describe a <strong>luciferin regenerating enzyme (LRE)</strong>, which we believed may solve these problems and allow brighter longer lasting light output without further addition of substrate. | [http://www.ncbi.nlm.nih.gov/pubmed/11457857 Gomi and Kajiyama (2001)] describe a <strong>luciferin regenerating enzyme (LRE)</strong>, which we believed may solve these problems and allow brighter longer lasting light output without further addition of substrate. | ||
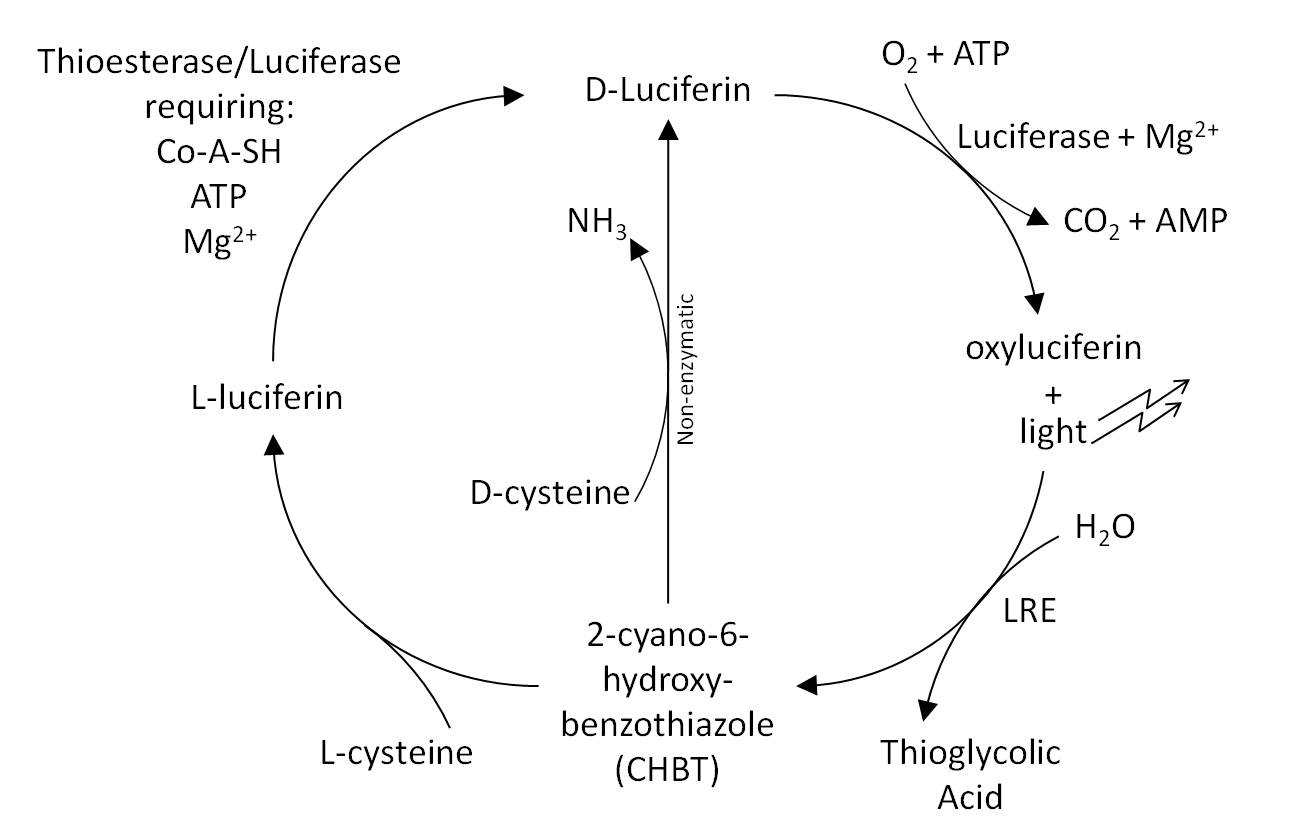
LRE transforms the inhibitory oxyluciferin to CHBT, which is converted back into the luciferin substrate required for light output. This was believed to occur in one of two ways: non-enzymatically requiring D-cysteine, or via L-luciferin requiring the L-cysteine. We aimed to verify these claims in our lab experiments and to quantify the difference in light output that LRE makes when expressed in ''E. coli''. To formally test the effect of the LRE we would have needed to add Oxyluciferin to the medium. Unfortunately, Oxyluciferin is not available commercially so we were not able to perform this experiment. We however tested other parts of the cycle, by adding CHBT and D-Cysteine to the medium. | LRE transforms the inhibitory oxyluciferin to CHBT, which is converted back into the luciferin substrate required for light output. This was believed to occur in one of two ways: non-enzymatically requiring D-cysteine, or via L-luciferin requiring the L-cysteine. We aimed to verify these claims in our lab experiments and to quantify the difference in light output that LRE makes when expressed in ''E. coli''. To formally test the effect of the LRE we would have needed to add Oxyluciferin to the medium. Unfortunately, Oxyluciferin is not available commercially so we were not able to perform this experiment. We however tested other parts of the cycle, by adding CHBT and D-Cysteine to the medium. | ||
| + | |||
[[Image:Cam-luci-cycle.jpg|center|500px]] | [[Image:Cam-luci-cycle.jpg|center|500px]] | ||
Latest revision as of 23:06, 27 October 2010

Introduction
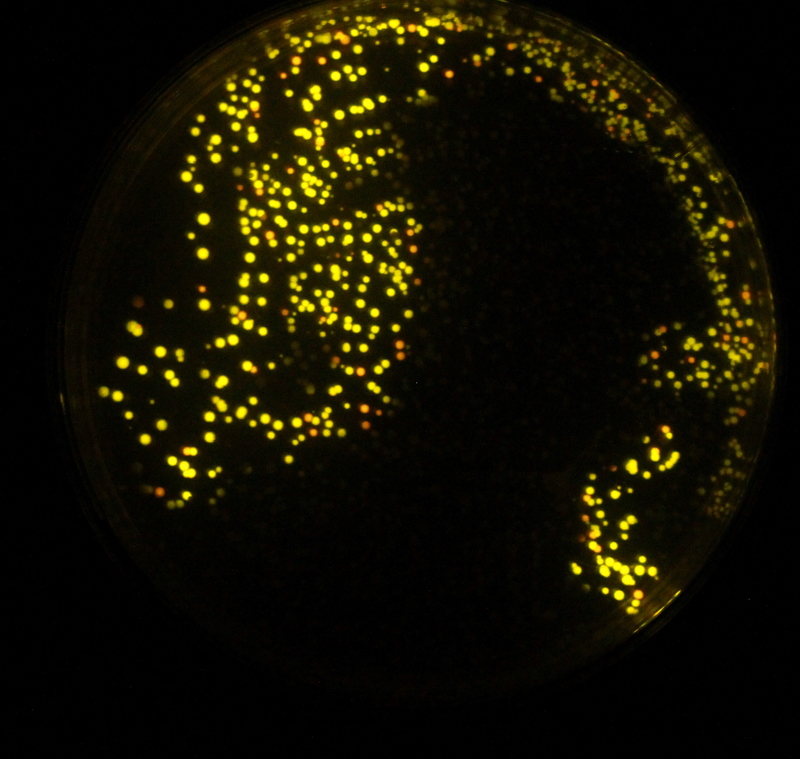
Fireflies are easily visible in the evening, whereas colonies of bacteria expressing luciferase are not. We hope to remove this divide by expressing further enzymes exploited by fireflies. In E. coli and in eukaryotes, each molecule of luciferin that emits light is turned into oxyluciferin and cannot be regenerated. A further problem is that this oxyluciferin inhibits the further reactions of luciferase.[http://www.ncbi.nlm.nih.gov/pubmed/11457857 Gomi and Kajiyama (2001)] describe a luciferin regenerating enzyme (LRE), which we believed may solve these problems and allow brighter longer lasting light output without further addition of substrate. LRE transforms the inhibitory oxyluciferin to CHBT, which is converted back into the luciferin substrate required for light output. This was believed to occur in one of two ways: non-enzymatically requiring D-cysteine, or via L-luciferin requiring the L-cysteine. We aimed to verify these claims in our lab experiments and to quantify the difference in light output that LRE makes when expressed in E. coli. To formally test the effect of the LRE we would have needed to add Oxyluciferin to the medium. Unfortunately, Oxyluciferin is not available commercially so we were not able to perform this experiment. We however tested other parts of the cycle, by adding CHBT and D-Cysteine to the medium.
Our Experiments
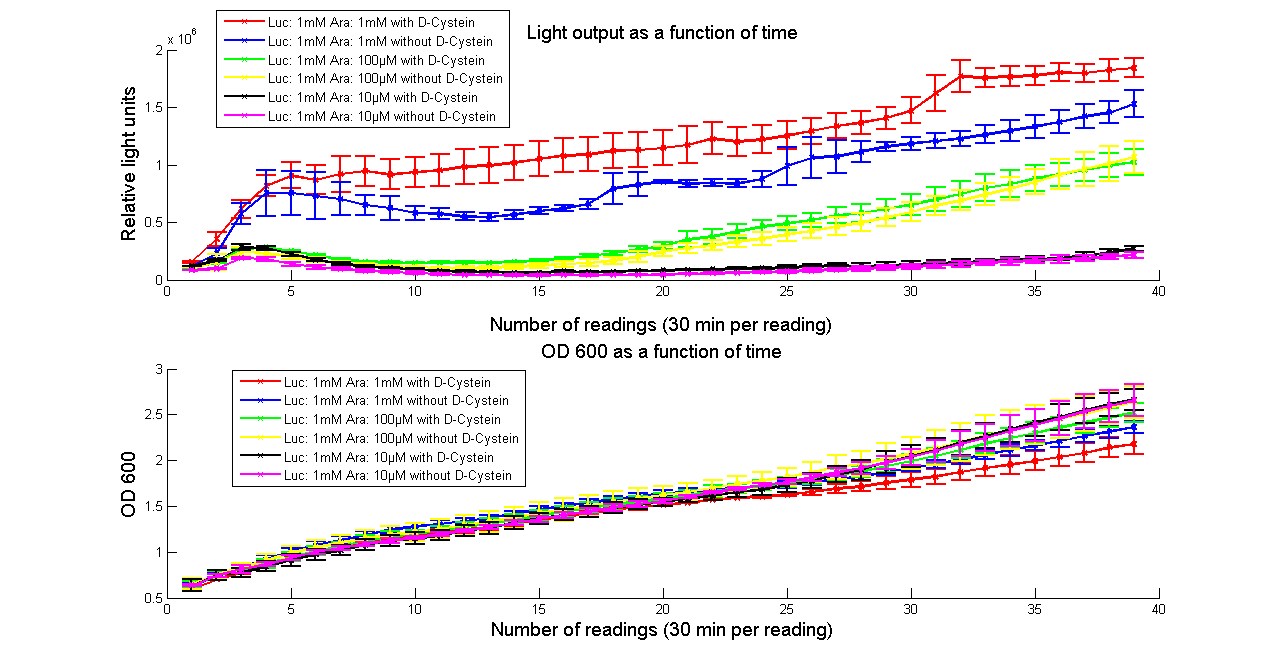
We tested both the L. cruciata and P. pyralis luciferases with and without LRE on our plate reader, kindly on loan from [http://www.bmglabtech.com/ BMG Labtech]. The results showed that when D-cysteine was added to the reaction, the light output was brighter and lasted for longer. This proved that both the working of LRE to produce CHBT and the conversion of CHBT into luciferin worked as expected.
However, the conversion from CHBT to luciferin via L-luciferin by adding L-cysteine was found not to work. Our research suggested that overexpression of thioesterase might help to make this possible, unfortunately our attempts to PCR out the E. coli thioesterase were not successful.
 "
"