Team:Cambridge/Bioluminescence/G28
From 2010.igem.org
| Line 1: | Line 1: | ||
{{:Team:Cambridge/Templates/headerMinimalprototype}} | {{:Team:Cambridge/Templates/headerMinimalprototype}} | ||
| - | {{:Team:Cambridge/Templates/headerbar|colour=#386abc| | + | {{:Team:Cambridge/Templates/headerbar|colour=#386abc|title=Project Vibrio: The LuxBrick}} |
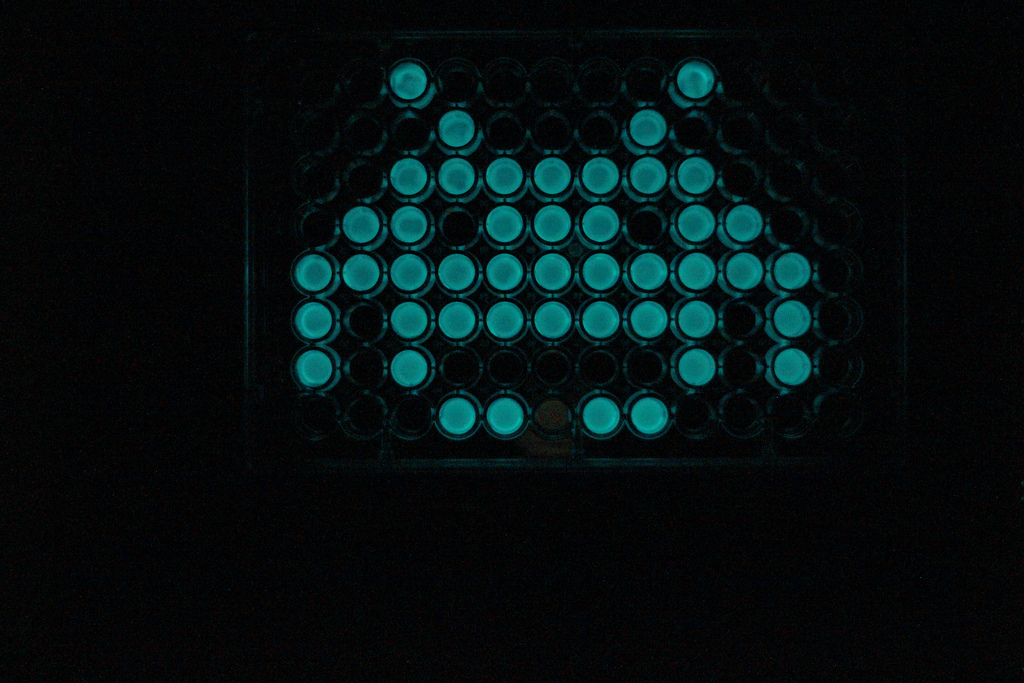
{{:Team:Cambridge/Templates/RightImage|image=Space_invader.jpg|caption=The LuxBrick on a 96 well plate}} | {{:Team:Cambridge/Templates/RightImage|image=Space_invader.jpg|caption=The LuxBrick on a 96 well plate}} | ||
Whilst researching the literature on bioluminescence at the beginning of the summer, we came across a report on [http://departments.kings.edu/biology/lux/bacterial.html 'Molecular Biology Experiments Utilizing the lux Genes of ''Vibrio fischeri'''] by James Slock from King's College, PA. Dr Slock kindly provided us the two plasmids mentioned in these experiments, which carry the complete ''V. fischeri'' lux operon (LuxICDABE regulated by Lux R). Using Long-Range PCR, we extracted luxCD, luxAB and luxE individually and assembled them into a new operon. As described in the [https://2010.igem.org/Team:Cambridge/Bioluminescence/Background Background section], lux I and lux R exert tight quorum sensing control on expression of the lux operon in ''V. fischeri''. In the absence of LuxR protein and AHL the Lux genes are virtually inactive. | Whilst researching the literature on bioluminescence at the beginning of the summer, we came across a report on [http://departments.kings.edu/biology/lux/bacterial.html 'Molecular Biology Experiments Utilizing the lux Genes of ''Vibrio fischeri'''] by James Slock from King's College, PA. Dr Slock kindly provided us the two plasmids mentioned in these experiments, which carry the complete ''V. fischeri'' lux operon (LuxICDABE regulated by Lux R). Using Long-Range PCR, we extracted luxCD, luxAB and luxE individually and assembled them into a new operon. As described in the [https://2010.igem.org/Team:Cambridge/Bioluminescence/Background Background section], lux I and lux R exert tight quorum sensing control on expression of the lux operon in ''V. fischeri''. In the absence of LuxR protein and AHL the Lux genes are virtually inactive. | ||
Latest revision as of 23:02, 27 October 2010

Whilst researching the literature on bioluminescence at the beginning of the summer, we came across a report on [http://departments.kings.edu/biology/lux/bacterial.html 'Molecular Biology Experiments Utilizing the lux Genes of Vibrio fischeri'] by James Slock from King's College, PA. Dr Slock kindly provided us the two plasmids mentioned in these experiments, which carry the complete V. fischeri lux operon (LuxICDABE regulated by Lux R). Using Long-Range PCR, we extracted luxCD, luxAB and luxE individually and assembled them into a new operon. As described in the Background section, lux I and lux R exert tight quorum sensing control on expression of the lux operon in V. fischeri. In the absence of LuxR protein and AHL the Lux genes are virtually inactive.
In order to relieve this control, we used Gibson Assembly to generate an operon consisting of Lux C, D, A, B, E (in this order, reflecting V. fischeri) under the arabinose-induced promoter pBAD ([http://partsregistry.org/Part:BBa_I0500 BBa_i0500]). We called this construct the LuxBrick. It caused bright and reproducible light output in the transformed E.coli Top10 cells. This new BioBrick ([http://partsregistry.org/Part:BBa_K325909 BBa_K325909]) can be used as an arabinose->light device and is a very useful part if the aim is to get a high bacterial light output. Many of the images in our Photo Gallery were created using Top10 cell transformed with this part.
h-ns mutants
To test the theory that H-NS proteins repress the Lux genes by interacting with their coding regions, we transformed two E.coli mutant strains with this construct and measured their light output. The strains we used were GM230 hns-205::Tn10, which has a C-terminal deletion in the H-NS gene and BW25113 Δhns::kan, a strain from a knockout library. In the literature, h-ns mutant strains have been described as producing much brighter luminescence than wild type strains.
While we could not reproduce a higher peak brightness, it was apparent that the knockout strain maintained its light output for much longer than wild type, which showed a steep reduction in brightness upon entering stationary phase. This phenomenon (Abrupt Decline in Luciferase Activity - or ADLA) has been described by [http://www.springerlink.com/content/w73k840k27866462/fulltext.pdf Koga et al. 2004]. However, this paper suggests that the effect of H-NS on luminescence is not due to expression of the lux genes, but occurs indirectly via the cell's redox pool, in particular the availability of FMNH2.
 "
"