Team:UNIPV-Pavia/Parts/Characterization/ExistingPartsRegistry
From 2010.igem.org
(→Existing Parts from the Registry: list) |
|||
| Line 43: | Line 43: | ||
---- | ---- | ||
<br> | <br> | ||
| + | |||
| + | =<partinfo>BBa_R0010</partinfo>, <partinfo>BBa_R0011</partinfo> - Wild type and hybrid lac promoters= | ||
| + | <partinfo>BBa_R0011</partinfo> hybrid lac promoter and the <partinfo>BBa_R0010</partinfo> wild type lac promoter were characterized at different copy number in TOP10 ''E. coli'' strain. This strain contains a lacI expression system in the genome. | ||
| + | |||
| + | Induction static transfer function (computed in Relative Promoter Units), dynamics and metabolic burden were evaluated as a function of different IPTG concentrations in M9 supplemented with glycerol growth medium. | ||
| + | |||
| + | A RFP generator (<partinfo>BBa_I13507</partinfo>) was used as a reporter gene. In particular, these measurement systems were used: | ||
| + | |||
| + | *<partinfo>pSB1A2</partinfo>-<partinfo>BBa_J107010</partinfo> | ||
| + | *<partinfo>pSB1A3</partinfo>-<partinfo>BBa_J04450</partinfo> | ||
| + | *<partinfo>pSB4C5</partinfo>-<partinfo>BBa_J107010</partinfo> | ||
| + | *<partinfo>pSB4C5</partinfo>-<partinfo>BBa_J04450</partinfo> | ||
| + | |||
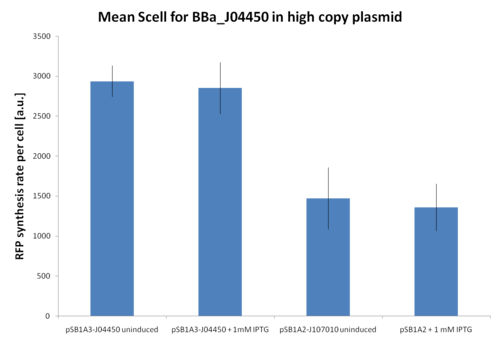
| + | At first, <partinfo>BBa_J107010</partinfo> and <partinfo>BBa_J04450</partinfo> inducibility was tested in a high copy number vector (<partinfo>pSB1A2</partinfo> or <partinfo>pSB1A3</partinfo>). The results are shown here as the relative RFP synthesis rate per cell. | ||
| + | |||
| + | |||
| + | {|align="center" | ||
| + | |[[Image:UNIPV_Pavia_r0010_1.png|500px|thumb|Relative RFP synthesis rate per cell in <partinfo>pSB1A2</partinfo>-<partinfo>BBa_J107010</partinfo> and <partinfo>pSB1A3</partinfo>-<partinfo>BBa_J04450</partinfo>. The error bars represent the standard errors of three independent measurements.]] | ||
| + | |} | ||
| + | |||
| + | |||
| + | Results show that in this condition <partinfo>BBa_R0010</partinfo> is about 2-fold stronger than <partinfo>BBa_R0011</partinfo>, but induced and uninduced cultures did not show differences in the RFP signal. | ||
| + | |||
| + | This result is expected because the vectors are propagated at about 200 copies per cell, while the lacI repressor is present at single copy in the genome and thus it is not able to repress the lac promoters in such high copy. | ||
| + | |||
| + | The doubling times and their standard errors estimated from data are reported below for <partinfo>pSB1A2</partinfo>-<partinfo>BBa_J107010</partinfo> and <partinfo>pSB1A3</partinfo>-<partinfo>BBa_J04450</partinfo> with and without 1mM of IPTG. | ||
| + | |||
| + | |||
| + | {| width='80%' align='center' border='1' | ||
| + | | '' Cultures'' || ''Mean doubling times [minutes]'' || ''standard errors over 3 independent experiment [minutes]'' | ||
| + | |- | ||
| + | | <partinfo>pSB1A2</partinfo>-<partinfo>BBa_J107010</partinfo> || 77,7 || 3,1 | ||
| + | |- | ||
| + | | <partinfo>pSB1A2</partinfo>-<partinfo>BBa_J107010</partinfo> + 1mM IPTG|| 76,5 || 2,2 | ||
| + | |- | ||
| + | | <partinfo>pSB1A3</partinfo>-<partinfo>BBa_J04450</partinfo>|| 107,8 || 0,3 | ||
| + | |- | ||
| + | | <partinfo>pSB1A3</partinfo>-<partinfo>BBa_J04450</partinfo> + 1mM IPTG|| 101,7 || 4,8 | ||
| + | |- | ||
| + | |} | ||
| + | |||
| + | |||
| + | These results demonstrate that cells growth is not significantly affected by the presence of IPTG, even at the high of 1 mM. | ||
| + | |||
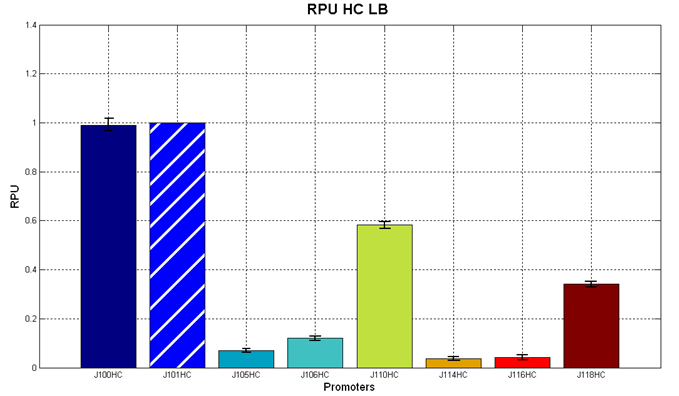
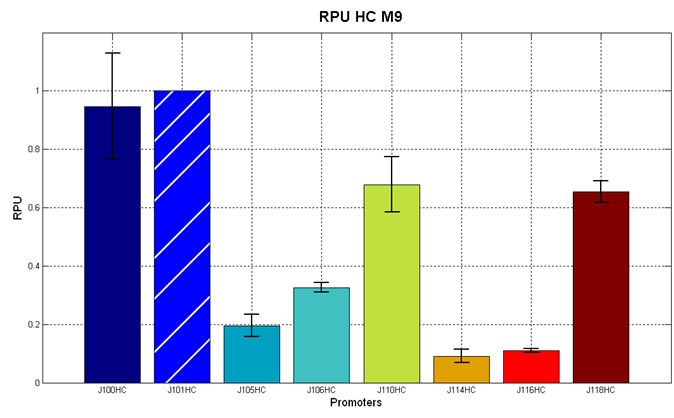
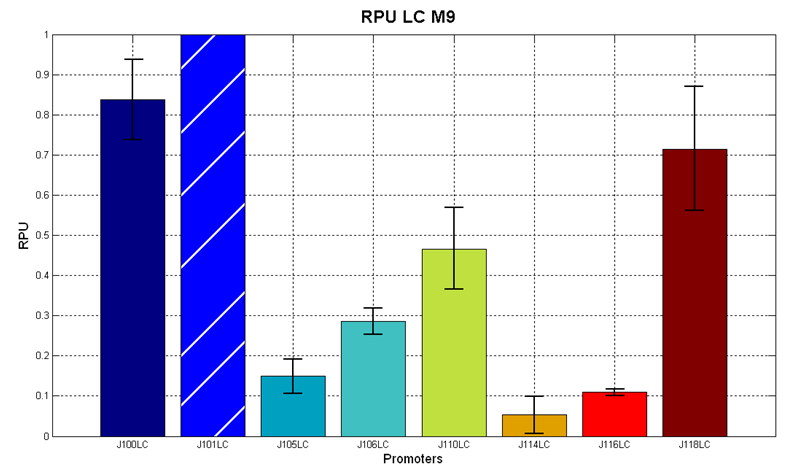
| + | <partinfo>BBa_J107010</partinfo> and <partinfo>BBa_J04450</partinfo> were then tested in the low copy (~5 copies per cell) vector <partinfo>pSB4C5</partinfo> in order to test their inducibility. The results are shown here as the RPU values at the steady state (constant RFP sysnthesis rate per cell) at different IPTG concentrations. | ||
| + | |||
| + | {|align="center" | ||
| + | |[[Image:UNIPV_Pavia_r0010_2.png|700px|thumb|RPU of <partinfo>pSB4C5</partinfo>-<partinfo>BBa_J107010</partinfo> and <partinfo>pSB4C5</partinfo>-<partinfo>BBa_J04450</partinfo> as a function of IPTG concentration. The error bars represent the standard errors of three independent measurements.]] | ||
| + | |} | ||
| + | |||
| + | Results show that in this condition both <partinfo>BBa_R0010</partinfo> and <partinfo>BBa_R0011</partinfo> produce different amounts of RFP as a function of the IPTG concentration. The amplitude of the two curves show that the promoters are very strong when induced with IPTG >= 10 uM. Although the experiments were carried out in the same conditions, the variability between experiments was high, especially for <partinfo>BBa_R0010</partinfo> (mean coefficient of variaton of about 37% for <partinfo>BBa_R0010</partinfo> and 15% for <partinfo>BBa_R0011</partinfo>), while the RPU variability between three wells in the same experiment is much lower (mean coefficient of variaton of bout 3.5% for both promoters). | ||
| + | |||
| + | The above figure shows that <partinfo>BBa_R0011</partinfo> is stronger than the <partinfo>BBa_R0010</partinfo> wild type promoter in low copy plasmid. This result is unexpected because the same promoters in high copy vectors behaved differently (<partinfo>BBa_R0010</partinfo> was stronger than the <partinfo>BBa_R0011</partinfo>, see above). | ||
| + | |||
| + | In the uninduced state, <partinfo>BBa_R0011</partinfo> has about the same strength as the <partinfo>BBa_J23101</partinfo> reference standard promoter. | ||
| + | This static characteristic shows that the promoters are both leaky and a very low IPTG concentration (10 uM) is sufficient to trigger gene expression at *very* high levels. | ||
| + | |||
| + | These results demonstrate that the genomic lacI is partially able to repress the two promoters, but very low IPTG concentrations are sufficient to bind the repressor and trigger the promoters transcription. | ||
| + | |||
| + | Doubling times were also estimated for these cultures. Their values are reported below for uninduced and 1 mM IPTG-induced cultures. | ||
| + | |||
| + | |||
| + | {| width='80%' align='center' border='1' | ||
| + | | '' Cultures'' || ''Mean doubling times [minutes]'' || ''standard errors over 3 independent experiments [minutes]'' | ||
| + | |- | ||
| + | | <partinfo>pSB4C5</partinfo>-<partinfo>BBa_J107010</partinfo> || 113,5 || 10,8 | ||
| + | |- | ||
| + | | <partinfo>pSB4C5</partinfo>-<partinfo>BBa_J107010</partinfo> + 1mM IPTG|| 106,8 || 5,5 | ||
| + | |- | ||
| + | | <partinfo>pSB4C5</partinfo>-<partinfo>BBa_J04450</partinfo>|| 85 || 5 | ||
| + | |- | ||
| + | | <partinfo>pSB4C5</partinfo>-<partinfo>BBa_J04450</partinfo> + 1mM IPTG|| 90 || 4,5 | ||
| + | |- | ||
| + | |} | ||
| + | |||
| + | |||
| + | As obtained for the cultures with high copy plasmids, the growth rate of TOP10 harbouring low copy vectors with the measurement parts is not affected by IPTG presence. | ||
| + | |||
| + | |||
| + | '''Dynamic characterization in low copy vector:''' The figure below shows a typical relative RFP synthesis rate per cell time series for <partinfo>BBa_J107010</partinfo> and <partinfo>BBa_J04450</partinfo> induced with 1 mM of IPTG and uninduced. These time series show that the full induction can be reached after about 50 min from the induction. | ||
| + | |||
| + | |||
| + | {|align="center" | ||
| + | |[[Image:UNIPV_Pavia_r0010_3.png|700px|thumb|Mean Scell signal as a function of time for <partinfo>pSB4C5</partinfo>-<partinfo>BBa_J107010</partinfo> and <partinfo>pSB4C5</partinfo>-<partinfo>BBa_J04450</partinfo>. Induced (with 1 mM of IPTG) and uninduced cultures are shown. Induction occurs at t=0. The shown graph is relative to one of the three experiments performed in different days.]] | ||
| + | |} | ||
| + | |||
| + | |||
| + | '''Conclusion:''' the characterization of two IPTG-inducible promoters has been performed and the performance of these two promoters have been compared in terms of transcriptional strength. The reported results are easily sharable in different laboratories thanks to the used standard RPU approach. | ||
| + | |||
| + | |||
| + | '''Methods:''' | ||
| + | *A of long term storage glycerol stock was streaked on a LB plate with suitable antibiotic. Tha plate was incubated overnight at 37°C. | ||
| + | *A single colony was inoculated in 1 ml of M9 + suitable antibiotic in a 15 ml tube and incubated at 37°C, 220 rpm for about 16 hours. | ||
| + | *The grown cultures were then diluted 1:100 in 2-5 ml of M9 supplemented medium and incubated in the same conditions as before for about 4-5 hours. | ||
| + | *For each desired IPTG concentration to be tested, three 200 ul aliquots of the cultures were aliquoted in a flat-bottom 96-well microplate, avoiding to perform dynamic experiments in the microplate frame (in order to prevent evaporation effects in the frame). | ||
| + | *2 ul of properly diluted IPTG (Sigma Aldrich) were added to the three wells for each desired concentration. | ||
| + | *The microplate was incubated in the Tecan Infinite F200 microplate reader and fluorescence and absorbance were measured with this automatic protocol: | ||
| + | **37°C constant for all the experiment; | ||
| + | **sampling time of 5 minutes; | ||
| + | **fluorescence gain of 50 or 80; | ||
| + | **O.D. filter at 600 nm; | ||
| + | **RFP filters at 535nm (ex) / 620nm (em); | ||
| + | **15 seconds of linear shaking (3mm amplitude) followed by 10 seconds of waiting before the measurements in order to make a homogeneous culture. | ||
| + | **Experiment duration time: about 6 hours. | ||
| + | *This experiment was performed three times in different days. | ||
| + | |||
| + | |||
| + | '''Data analysis:''' Relative Promoter Units (RPUs) were estimated as described by [Kelly JR et al. (2009), J Biol Eng 3:4]. | ||
| + | |||
| + | Briefly: | ||
| + | *Absorbance and fluorescence time series were normalized by subtracting the absorbance of the media and the fluorescence of a negative control (a non fluorescent TOP10 culture) respectively, thus yielding O.D.600 and RFP time series. | ||
| + | *RFP synthesis rate per cell (called ''Scell'') was computed as (1/O.D.600)*dGFP/dt. (this signal is not actually the RFP synthesis rate, but is proportional to it). | ||
| + | *The RFP synthesis rate per cell was averaged at the steady state during the exponential growth phase (validated by identifying the linear region of the ln(O.D.600)). | ||
| + | *The RPU of the promoter of interest in a specific condition was computed as ''mean_Scell,phi/mean_Scell,J23101'' where phi is the promoter of interest, J23101 is the reference standard and ''mean_Scell'' is the mean Scell signal value, computed as explained above. | ||
| + | |||
=<partinfo>BBa_K300009</partinfo>/<partinfo>BBa_I4102</partinfo> - PoPS->3OC6HSL sender device= | =<partinfo>BBa_K300009</partinfo>/<partinfo>BBa_I4102</partinfo> - PoPS->3OC6HSL sender device= | ||
Revision as of 22:37, 27 October 2010
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 "
"