Team:NYMU-Taipei/Experiments/Speedy switch
From 2010.igem.org
(→Repoting Assay4) |
(→Reporting Assay1) |
||
| Line 37: | Line 37: | ||
[[Image:Plot20101023.png|800px]][fig.2] | [[Image:Plot20101023.png|800px]][fig.2] | ||
*Discussion | *Discussion | ||
| - | Under 4mM theophylline , we added more concentrations of theophylline the protein expressed stronger ,so we can see the higher fluorescent intensity . From the paper said if added more than 5mM theophylline the | + | **Under 4mM theophylline , we added more concentrations of theophylline the protein expressed stronger ,so we can see the higher fluorescent intensity . From the paper said if added more than 5mM theophylline the ''escherichia coli'' would die. |
| + | **The 8mM and 10mM lines were low initially and then growing.We sugested that may have two possibility.One is that because we take out ''escherichia coli'' from 37 degrees centigrade and added theophylline in it at room temperature and we take the ''escherichia coli'' into 37 degrees centigrade incubator.So the ''escherichia coli'' may not get with the temperature and die initially. | ||
==Reporting Assay 2== | ==Reporting Assay 2== | ||
Revision as of 18:32, 27 October 2010
| Home | Project Overview | Speedy reporter | Speedy switch | Speedy protein degrader | Experiments and Parts | Applications | F.A.Q | About Us |
Contents |
Method
- Protocol:
1.Selected genes to be reported are incubated overnight in an LB liquid culture at 37oC and 180-200rpm. This makes sure they are fresh in the morning. Positive and negative controls are also needed.
2.Overnight liquid culture is diluted to OD600 of 0.1, Theophylline is added at concentrations ranging from 0.01mM to 20mM, and the mix incubated for 2-2.5 hours.
3.Measurement of OD at 2 hours: For each used well in the 96-well plate: Take 200uL from the liquid (make sure you pipette this step well) and put it in a cuvette to read the OD600. Note down the OD600 ["OD at 2 hours"], then take the liquid in the cuvette and put it in the right place in the 96-well plate.
4.Measurement of fluorescence: Continuous measurement of fluorescence with the excitation/emission wavelengths 488/511nm for 2 hours, with one fluorescence data point every 2 minutes.
5.Measurement of OD at 4 hours: For each used well in the 96-well plate: Take the liquid from the well and put it in the cuvette to measure the OD ["OD at 4 hours"].
- The optimizing data:
We took the data from OD600 of each sample, which should have an exponential growth curve, and took the ln of each value. After taking the logarithm of the data, we created a linear curve. Since we have the two end points of the OD 600 of each sample, we use this linear curve to modulate OD value of each sample at each specific time point. This value was then recalculated back into its original curve using exponents. Our fluorescent data was normalized by taking the fluorescence of our sample at each time point and subtracting the fluorescence of the negative control in the same OD value at the same time point. Finally, plot the nomalized fluorescence versus time in minutes scale.
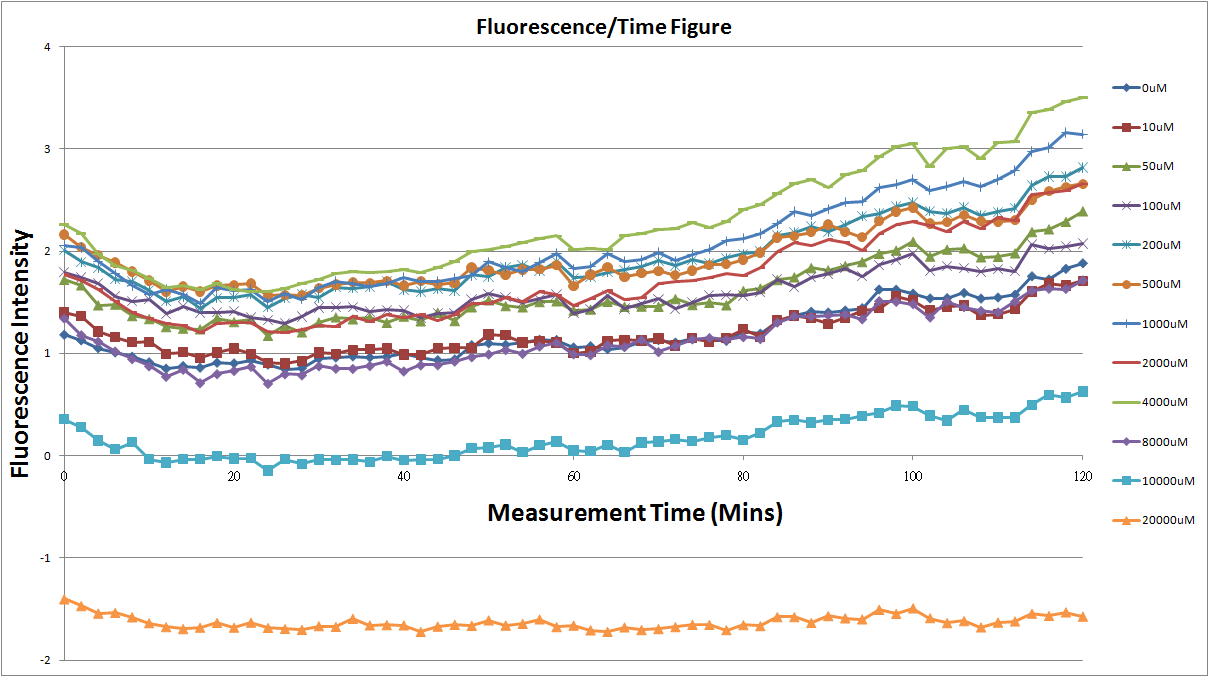
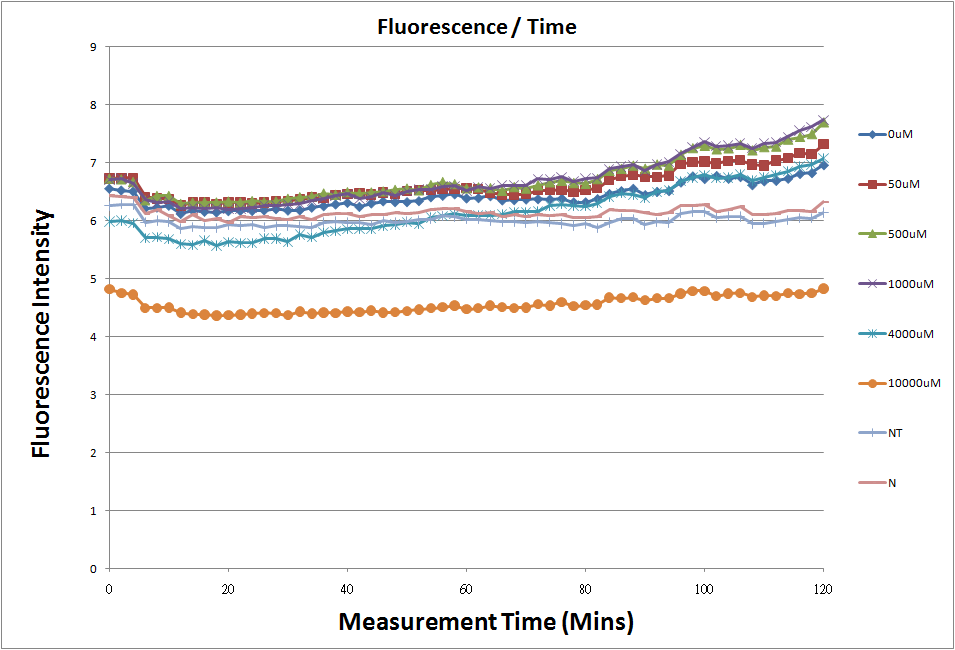
Reporting Assay
- Fig.1, fig.3, fig.5, and fig.7 are the original charts of the experiment. N line and NT line are control line. N line stands for the cell which sequence doesn't have pLac promoter.NT line stands for the cell which sequence doesn't have promoter but added 0.1mM Theophylline. N line shows that even though the sequence doesn't have promoter it still have little fluorescence so we use it to modify the instrument errors. 0μM sample which sequence has promoter but doesn’t added Theophylline still has little fluorescence because mRNA is leak. Normalizing with the N, we use this line to find out what's the degree of mRNA leak. NT line which doesn’t have promoter in the plasmid but add 0.1 mM Theophylline still is detected fluorescence. We added Theophylline in DMSO, so we use this NT line to normalize the other samples which added different concentration of Theophylline in order to eliminate the spectrum effect of Theophylline.
- Fig.2, fig.4, fig.6, and fig.8 are charts which have been normalized with the control N line and NT line.
Reporting Assay 1
- The oringinal fluorescence data.
- Normalized the 12 different Theophylline concentration samples with NT & N.
- Discussion
- Under 4mM theophylline , we added more concentrations of theophylline the protein expressed stronger ,so we can see the higher fluorescent intensity . From the paper said if added more than 5mM theophylline the escherichia coli would die.
- The 8mM and 10mM lines were low initially and then growing.We sugested that may have two possibility.One is that because we take out escherichia coli from 37 degrees centigrade and added theophylline in it at room temperature and we take the escherichia coli into 37 degrees centigrade incubator.So the escherichia coli may not get with the temperature and die initially.
Reporting Assay 2
- The original fluorescence data.
- Normalized 12 different concentration of Theophylline with NT & N.
Reporting Assay 3
- The oringinal fluorescence data.
- Normalized the 6 different Theophylline concentration samples with NT & N.
Repoting Assay 4
- [Reagent Formula]
- The original fluorescence data.
- Normalized 6 different concentration of Theophylline with NT & N.
 "
"