Team:NYMU-Taipei/Project/Speedy protein degrader
From 2010.igem.org
(→Reference) |
(→Sequences) |
||
| Line 110: | Line 110: | ||
=<font color=blue>Sequences</font>= | =<font color=blue>Sequences</font>= | ||
{{:Team:NYMU-Taipei/Footer}} | {{:Team:NYMU-Taipei/Footer}} | ||
| + | == Project overview == | ||
| + | A major goal of synthetic biology is to design and build custom signaling circuits, which can monitor internal and external states, with available molecular tools. To achieve a dynamic response and a quick adaptation to signals, we try to control the half-life of proteins by proteolysis. | ||
| + | |||
| + | == SsrA tags == | ||
| + | SSrA degradation tags to use: | ||
| + | {| border="1" | ||
| + | | Tag || Amino Acid || Sequence | ||
| + | |- | ||
| + | | [ LVA] || RPAANDENYALVA || gctgcaaacgacgaaaactacgctttagtagct | ||
| + | |- | ||
| + | | [http://partsregistry.org/wiki/index.php/Part:BBa_M0050 LAA] || AANDENYALAA || gctgctaacgacgaaaactacgctctggctgct | ||
| + | |} | ||
| + | |||
| + | There were Biobricks for these two tags. | ||
| + | BBa_M0040 for the LVA tag and BBa_M0042 for the LAA tag, but they are all "planning". | ||
| + | |||
| + | So I am wondering that if we can design or make this two parts for the iGEM? | ||
| + | |||
| + | == Fluorescent proteins == | ||
| + | {{:Team:NYMU-Taipei/NStyle}} | ||
| + | |||
| + | Fluorescent proteins to add degradation tags onto. | ||
| + | * [http://openwetware.org/wiki/IGEM:IMPERIAL/2007/Projects/Reporters#bibkey_Andersen FP comparison] | ||
| + | ** Maturation time is the time until the protein starts shining. | ||
| + | |||
| + | === GFP === | ||
| + | <!-- | ||
| + | * [http://partsregistry.org/wiki/index.php/Part:BBa_E0040 GFP coding region]. | ||
| + | * [http://partsregistry.org/wiki/index.php/Part:BBa_E0240 GFP generator 1] (B0032+E0040+B0015). | ||
| + | * [http://partsregistry.org/wiki/index.php/Part:BBa_I732077 GFP generator 2] (B0034+J04031+B0015). | ||
| + | * [http://partsregistry.org/wiki/index.php/Part:BBa_I732077 GFP generator 3] (B0034+J04031+B0015). | ||
| + | * [http://partsregistry.org/wiki/index.php/Part:BBa_K145015 GFP with LVA tag 1] | ||
| + | * Anymore similar things in parts registry? | ||
| + | * And please help check about the links to those biobricks or parts. | ||
| + | --> | ||
| + | * Used [http://partsregistry.org/wiki/index.php/Part:BBa_E0240 BBa_E0240] as a template: | ||
| + | {{:Team:NYMU-Taipei/Seq|w=720|[http://partsregistry.org/Part:BBa_E0240 BBa_E0240]|876|{{:Team:NYMU-Taipei/N|R|tcacacaggaaag}}tactag{{:Team:NYMU-Taipei/N|C|atgcgtaaaggagaagaacttttcactggagttgtcccaattcttgttgaattagatggtgatgttaatgggcacaaattt | ||
| + | tctgtcagtggagagggtgaaggtgatgcaacatacggaaaacttacccttaaatttatttgcactactggaaaactacctgttccatggccaacacttg | ||
| + | tcactactttcggttatggtgttcaatgctttgcgagatacccagatcatatgaaacagcatgactttttcaagagtgccatgcccgaaggttatgtaca | ||
| + | ggaaagaactatatttttcaaagatgacgggaactacaagacacgtgctgaagtcaagtttgaaggtgatacccttgttaatagaatcgagttaaaaggt | ||
| + | attgattttaaagaagatggaaacattcttggacacaaattggaatacaactataactcacacaatgtatacatcatggcagacaaacaaaagaatggaa | ||
| + | tcaaagttaacttcaaaattagacacaacattgaagatggaagcgttcaactagcagaccattatcaacaaaatactccaattggcgatggccctgtcct | ||
| + | tttaccagacaaccattacctgtccacacaatctgccctttcgaaagatcccaacgaaaagagagaccacatggtccttcttgagtttgtaacagctgct | ||
| + | gggattacacatggcatggatgaactatacaaataataa}}tactagag{{:Team:NYMU-Taipei/N|T|ccaggcatcaaataaaacgaaaggctcagtcgaaagactgggcctttcgtttt | ||
| + | atctgttgtttgtcggtgaacgctctctactagagtcacactggctcaccttcgggtgggcctttctgcgtttata}}}} | ||
| + | |||
| + | BBa_E0240+LVA (note LVA was added before the taataa double-stop codon) | ||
| + | {{:Team:NYMU-Taipei/Seq|w=720|BBa_E0240+LVA|909|{{:Team:NYMU-Taipei/N|R|tcacacaggaaag}}tactag{{:Team:NYMU-Taipei/N|C|atgcgtaaaggagaagaacttttcactggagttgtcccaattcttgttgaattagatggtgatgttaatgggcacaaattt | ||
| + | tctgtcagtggagagggtgaaggtgatgcaacatacggaaaacttacccttaaatttatttgcactactggaaaactacctgttccatggccaacacttg | ||
| + | tcactactttcggttatggtgttcaatgctttgcgagatacccagatcatatgaaacagcatgactttttcaagagtgccatgcccgaaggttatgtaca | ||
| + | ggaaagaactatatttttcaaagatgacgggaactacaagacacgtgctgaagtcaagtttgaaggtgatacccttgttaatagaatcgagttaaaaggt | ||
| + | attgattttaaagaagatggaaacattcttggacacaaattggaatacaactataactcacacaatgtatacatcatggcagacaaacaaaagaatggaa | ||
| + | tcaaagttaacttcaaaattagacacaacattgaagatggaagcgttcaactagcagaccattatcaacaaaatactccaattggcgatggccctgtcct | ||
| + | tttaccagacaaccattacctgtccacacaatctgccctttcgaaagatcccaacgaaaagagagaccacatggtccttcttgagtttgtaacagctgct | ||
| + | gggattacacatggcatggatgaactatacaaa{{:Team:NYMU-Taipei/N|S|GCTGCAAACGACGAAAACTACGCTTTAGTAGCT}}taataa}}tactagag{{:Team:NYMU-Taipei/N|T|ccaggcatcaaataaaacgaaaggctcagtcgaaagactgggcctttcgtttt | ||
| + | atctgttgtttgtcggtgaacgctctctactagagtcacactggctcaccttcgggtgggcctttctgcgtttata}}}} | ||
| + | |||
| + | Primers designed by Jesse in iGEM2008. | ||
| + | {{:Team:NYMU-Taipei/Seq|w=720|BBa_E0240+LVA|909|{{:Team:NYMU-Taipei/N|FP|tcacacaggaaagtactagatgc}}gtaaaggagaagaacttttcactggagttgtcccaattcttgttgaattagatggtgatgttaatgggcacaaattt | ||
| + | tctgtcagtggagagggtgaaggtgatgcaacatacggaaaacttacccttaaatttatttgcactactggaaaactacctgttccatggccaacacttg | ||
| + | tcactactttcggttatggtgttcaatgctttgcgagatacccagatcatatgaaacagcatgactttttcaagagtgccatgcccgaaggttatgtaca | ||
| + | ggaaagaactatatttttcaaagatgacgggaactacaagacacgtgctgaagtcaagtttgaaggtgatacccttgttaatagaatcgagttaaaaggt | ||
| + | attgattttaaagaagatggaaacattcttggacacaaattggaatacaactataactcacacaatgtatacatcatggcagacaaacaaaagaatggaa | ||
| + | tcaaagttaacttcaaaattagacacaacattgaagatggaagcgttcaactagcagaccattatcaacaaaatactccaattggcgatggccctgtcct | ||
| + | tttaccagacaaccattacctgtccacacaatctgccctttcgaaagatcccaacgaaaagagagaccacatggtccttcttgagtttgtaacagctgct | ||
| + | gggattaca{{:Team:NYMU-Taipei/N|FP|catggcatggatgaactatacaaaGCTGCAAA}}{{:Team:NYMU-Taipei/N|OP|CGACGAAAACTACGCTT}}{{:Team:NYMU-Taipei/N|FP|TAGTAGCTtaataatactagagccaggcatca}}aataaaacgaaaggctcagtcgaaagactgggcctttcgtttt | ||
| + | atctgttgtttgtcggtgaacgctctctactagagtcacactggctcaccttcggg{{:Team:NYMU-Taipei/N|RP|tgggcctttctgcgtttata}}}} | ||
| + | |||
| + | <pre> | ||
| + | Primers: (directly copied from iGEM2008 wiki.) | ||
| + | Outer primers: | ||
| + | gaattcgcggccgcttctagag tcacacaggaaagtactagatgc (prefix + anneal: len:23, tm:56, %gc:43) | ||
| + | ctgcagcggccgctactagta ataaacgcagaaaggccca (suffix + anneal: len:19, tm:56, %gc:47) | ||
| + | Inner primers: | ||
| + | Forward: CGACGAAAACTACGCTTTAGTAGCT taataatactagagccaggcatca (insert + anneal: len:24, tm:55, %gc38) | ||
| + | Reverse: AAGCGTAGTTTTCGTCGTTTGCAGC tttgtatagttcatccatgccatg (insert + anneal: len:24, tm:55, %gc:38) | ||
| + | Complimented part: CGACGAAAACTACGCTT (len:17, tm:50, %gc:47) | ||
| + | </pre> | ||
| + | |||
| + | Final product is a Linear PCR DNA containing E0240+LVA. | ||
| + | |||
| + | Redesign Outer FP to include B0034 instead of B0032 (Lets save a cloning cycle) | ||
| + | <pre> | ||
| + | XbaI+B0034+scar+E0040 start | ||
| + | gcttctagag aaagaggagaaa tactag atgcgtaaaggagaagaacttttc (55C,24bp,38%GC) Total:52bp | ||
| + | </pre> | ||
| + | |||
| + | === RFP === | ||
| + | PCR of [http://partsregistry.org/Part:BBa_I13507 BBa_I13507] | ||
| + | {{:Team:NYMU-Taipei/Seq|[http://partsregistry.org/Part:BBa_I13507 BBa_I13507]|861|{{:Team:NYMU-Taipei/SeqPart|R|aaagaggagaaa}}tactag{{:Team:NYMU-Taipei/SeqPart|C|atggcttcctccgaagacgttatcaaagagttcatgcgtttcaaagttcgtatggaaggttccgttaacggtcacgagttcg | ||
| + | aaatcgaaggtgaaggtgaaggtcgtccgtacgaaggtacccagaccgctaaactgaaagttaccaaaggtggtccgctgccgttcgcttgggacatcct | ||
| + | gtccccgcagttccagtacggttccaaagcttacgttaaacacccggctgacatcccggactacctgaaactgtccttcccggaaggtttcaaatgggaa | ||
| + | cgtgttatgaacttcgaagacggtggtgttgttaccgttacccaggactcctccctgcaagacggtgagttcatctacaaagttaaactgcgtggtacca | ||
| + | acttcccgtccgacggtccggttatgcagaaaaaaaccatgggttgggaagcttccaccgaacgtatgtacccggaagacggtgctctgaaaggtgaaat | ||
| + | caaaatgcgtctgaaactgaaagacggtggtcactacgacgctgaagttaaaaccacctacatggctaaaaaaccggttcagctgccgggtgcttacaaa | ||
| + | accgacatcaaactggacatcacctcccacaacgaagactacaccatcgttgaacagtacgaacgtgctgaaggtcgtcactccaccggtgcttaataa}}c | ||
| + | gctgatagtgctagtgtagatcgctactagag{{:Team:NYMU-Taipei/SeqPart|T|ccaggcatcaaataaaacgaaaggctcagtcgaaagactgggcctttcgttttatctgttgtttgtcg | ||
| + | gtgaacgctctctactagagtcacactggctcaccttcgggtgggcctttctgcgtttata}}}} | ||
| + | What's with the extra long scar after the double translational start codon? '''Lets ignore it.''' (someone identify it please) | ||
| + | {{:Team:NYMU-Taipei/Seq|[http://partsregistry.org/Part:BBa_I13507 BBa_I13507]+LVA|869|{{:Team:NYMU-Taipei/SeqPart|R|aaagaggagaaa}}tactag{{:Team:NYMU-Taipei/SeqPart|C|atggcttcctccgaagacgttatcaaagagttcatgcgtttcaaagttcgtatggaaggttccgttaacggtcacgagttcg | ||
| + | aaatcgaaggtgaaggtgaaggtcgtccgtacgaaggtacccagaccgctaaactgaaagttaccaaaggtggtccgctgccgttcgcttgggacatcct | ||
| + | gtccccgcagttccagtacggttccaaagcttacgttaaacacccggctgacatcccggactacctgaaactgtccttcccggaaggtttcaaatgggaa | ||
| + | cgtgttatgaacttcgaagacggtggtgttgttaccgttacccaggactcctccctgcaagacggtgagttcatctacaaagttaaactgcgtggtacca | ||
| + | acttcccgtccgacggtccggttatgcagaaaaaaaccatgggttgggaagcttccaccgaacgtatgtacccggaagacggtgctctgaaaggtgaaat | ||
| + | caaaatgcgtctgaaactgaaagacggtggtcactacgacgctgaagttaaaaccacctacatggctaaaaaaccggttcagctgccgggtgcttacaaa | ||
| + | accgacatcaaactggacatcacctcccacaacgaagactacaccatcgttgaacagtacgaacgtgctgaaggtcgtcactccaccggtgct{{:Team:NYMU-Taipei/SeqPart|S|GCTGCAA | ||
| + | ACGACGAAAACTACGCTTTAGTAGCT}}taataa}}tactagag{{:Team:NYMU-Taipei/SeqPart|T|ccaggcatcaaataaaacgaaaggctcagtcgaaagactgggcctttcgttttatctgtt | ||
| + | gtttgtcggtgaacgctctctactagagtcacactggctcaccttcgggtgggcctttctgcgtttata}}}} | ||
| + | Primers: | ||
| + | {{:Team:NYMU-Taipei/Seq|[http://partsregistry.org/Part:BBa_I13507 BBa_I13507]+LVA|869|{{:Team:NYMU-Taipei/N|FP|{{:Team:NYMU-Taipei/N|R|aaagaggagaaa}}tactag{{:Team:NYMU-Taipei/N|C|atggct}}}}{{:Team:NYMU-Taipei/N|C|tcctccgaagacgttatcaaagagttcatgcgtttcaaagttcgtatggaaggttccgttaacggtcacgagttcg | ||
| + | aaatcgaaggtgaaggtgaaggtcgtccgtacgaaggtacccagaccgctaaactgaaagttaccaaaggtggtccgctgccgttcgcttgggacatcct | ||
| + | gtccccgcagttccagtacggttccaaagcttacgttaaacacccggctgacatcccggactacctgaaactgtccttcccggaaggtttcaaatgggaa | ||
| + | cgtgttatgaacttcgaagacggtggtgttgttaccgttacccaggactcctccctgcaagacggtgagttcatctacaaagttaaactgcgtggtacca | ||
| + | acttcccgtccgacggtccggttatgcagaaaaaaaccatgggttgggaagcttccaccgaacgtatgtacccggaagacggtgctctgaaaggtgaaat | ||
| + | caaaatgcgtctgaaactgaaagacggtggtcactacgacgctgaagttaaaaccacctacatggctaaaaaaccggttcagctgccgggtgcttacaaa | ||
| + | accgacatcaaactggacatcacctcccacaacgaagactacaccatcgttgaacagtacgaacgtgctgaaggtcg{{:Team:NYMU-Taipei/N|RP|tcactccaccggtgctGCTGCAA | ||
| + | A}}{{:Team:NYMU-Taipei/N|OP|CGACGAAAACTACGCTTTAGTAG}}}}{{:Team:NYMU-Taipei/N|FP|{{:Team:NYMU-Taipei/N|C|CTtaataa}}tactagag{{:Team:NYMU-Taipei/N|T|ccaggcatca}}}}{{:Team:NYMU-Taipei/N|T|aataaaacgaaaggctcagtcgaaagactgggcctttcgttttatctgtt | ||
| + | gtttgtcggtgaacgctctctactagagtcacactggctcaccttcggg{{:Team:NYMU-Taipei/N|RP|tgggcctttctgcgtttata}}}}}} | ||
| + | |||
| + | |||
| + | <pre> | ||
| + | Primers to order: | ||
| + | Outer FP: gaattcgcggccgcttctagag aaagaggagaaatactagatggct (55C,24bp,38%GC) Total: | ||
| + | Inner RP: CTACTAAAGCGTAGTTTTCGTCGTTTGCAGCagcaccggtggagtga (56C,16bp,63%GC) Total:47bp | ||
| + | Primers that are the same as GFP: | ||
| + | Inner FP: CGACGAAAACTACGCTTTAGTAGCTtaataatactagagccaggcatca (55C,24bp,38%GC) Total:49bp | ||
| + | Outer RP: ctgcagcggccgctactagta tataaacgcagaaaggccca (56C,20bp,45%GC) Total:41bp | ||
| + | Overlapping region: 55C,23bp,43%GC | ||
| + | </pre> | ||
| + | |||
| + | === YFP === | ||
| + | <!-- | ||
| + | * [http://partsregistry.org/Part:BBa_E0430 YFP-LVA] Why give a non-LVA one? | ||
| + | * [http://partsregistry.org/Part:BBa_E0438 YFP+LVA], contains the LVA sequence, however DNA is unavailable | ||
| + | {{:Team:NYMU-Taipei/Seq|w=720|[http://partsregistry.org/Part:BBa_E0438 BBa_E0430]|883|{{:Team:NYMU-Taipei/SeqPart|R|aaagaggagaaa}}tactag{{:Team:NYMU-Taipei/SeqPart|C|atggtgagcaagggcgaggagctgttcaccggggtggtgcccatcctggtcgagctggacggcgacgtaaacggccacaagt | ||
| + | tcagcgtgtccggcgagggcgagggcgatgccacctacggcaagctgaccctgaagttcatctgcaccaccggcaagctgcccgtgccctggcccaccct | ||
| + | cgtgaccaccttcggctacggcctgcaatgcttcgcccgctaccccgaccacatgaagctgcacgacttcttcaagtccgccatgcccgaaggctacgtc | ||
| + | caggagcgcaccatcttcttcaaggacgacggcaactacaagacccgcgccgaggtgaagttcgagggcgacaccctggtgaaccgcatcgagctgaagg | ||
| + | gcatcgacttcaaggaggacggcaacatcctggggcacaagctggagtacaactacaacagccacaacgtctatatcatggccgacaagcagaagaacgg | ||
| + | catcaaggtgaacttcaagatccgccacaacatcgaggacggcagcgtgcagctcgccgaccactaccagcagaacacccccatcggcgacggccccgtg | ||
| + | ctgctgcccgacaaccactacctgagctaccagtccgccctgagcaaagaccccaacgagaagcgcgatcacatggtcctgctggagttcgtgaccgccg | ||
| + | ccgggatcactctcggcatggacgagctgtacaagaggcct{{:Team:NYMU-Taipei/SeqPart|S|gctgcaaacgacgaaaactacgctttagtagct}}taataa}}tactagag{{:Team:NYMU-Taipei/SeqPart|T|tcacactggctc | ||
| + | accttcgggtgggcctttctgcgtttatatactagagagagaatataaaaagccagattattaatccggcttttttattattt}}}} | ||
| + | --> | ||
| + | * [http://partsregistry.org/wiki/index.php/Part:BBa_E0032 YFP with LVA tag] | ||
| + | |||
| + | === CFP === | ||
| + | |||
| + | * [http://partsregistry.org/wiki/index.php/Part:BBa_E0022 CFP with LVA tag] | ||
| + | |||
| + | == Degradation related E. coli proteins == | ||
| + | * "[http://www.nature.com/nsmb/journal/v10/n9/full/nsb0903-676.html A tag and enhances its recognition by ClpXP.]" (Jabri03, Nature) | ||
| + | * [http://biocyc.org/ECOLI/NEW-IMAGE?type=ENZYME&object=CPLX0-3107 ClpXP degrades a whole range of proteins]. Does this mean that overexpression will kill ''E. coli'' due to it killing a lot more proteins than it suppose to?? | ||
| + | * [http://www.cell.com/molecular-cell/retrieve/pii/S1097276504005908 A protein that has been covalently linked to SspB becomes a ClpXP substrate even in the absence of an SsrA tag]. (Bolon04, Cell) | ||
| + | ** Does this mean that overexpression of SspB will kill ''E. coli'' due to it binding on different proteins? | ||
| + | === ClpX === | ||
| + | === ClpP === | ||
| + | === SspB === | ||
| + | * [http://biocyc.org/ECOLI/NEW-IMAGE?type=GENE&object=EG10978 Ecocyc] link. | ||
| + | * [http://biocyc.org/ECOLI/sequence-rc?type=GENE&object=EG10978 SspB] sequence. | ||
| + | {{:Team:NYMU-Taipei/Seq|w=720|SspB|498|{{:Team:NYMU-Taipei/SeqPart|C|{{:Team:NYMU-Taipei/N|FP|atgGATTTGT CACAGCTAAC AC}}CACGTCGT CCCTATCTGC TGCGTGCATT CTATGAGTGG | ||
| + | TTGCTGGATA ACCAGCTCAC GCCGCACCTG GTGGTGGATG TGACGCTCCC TGGCGTGCAG | ||
| + | GTTCCTATGG AATATGCGCG TGACGGGCAA ATCGTACTCA ACATTGCGCC GCGTGCTGTC | ||
| + | GGCAATCTGG AACTGGCGAA TGATGAGGTG CGCTTTAACG CGCGCTTTGG TGGCATTCCG | ||
| + | CGTCAGGTTT CTGTGCCGCT GGCTGCCGTG CTGGCTATCT ACGCCCGTGA AAATGGCGCA | ||
| + | GGCACGATGT TTGAGCCTGA AGCTGCCTAC GATGAAGATA CCAGCATCAT GAATGATGAA | ||
| + | GAGGCATCGG CAGACAACGA AACCGTTATG TCGGTTATTG ATGGCGACAA GCCAGATCAC | ||
| + | GATGATGACA CTCATCCTGA CGATGAACCT CCGCAGCCAC CACGCGGTGG TCGACCG{{:Team:NYMU-Taipei/N|RP|GCA | ||
| + | TTACGCGTTG TGAAGta}}}}}} | ||
| + | <pre> | ||
| + | FP: gcttctag atgGATTTGTCACAGCTAACAC temp:55C, len:22bp, %GC:41% (total 30bp) | ||
| + | RP: ctgcagcggccgctactagta ttaCTTCACAACGCGTAATGC temp:55C, len:21bp, %GC:43% (total 41bp) | ||
| + | </pre> | ||
| + | |||
| + | == Measurements == | ||
| + | * [http://www.ncbi.nlm.nih.gov/pmc/articles/PMC106306/?tool=pubmed one to follow] unless there's a better one [https://2008.igem.org/Team:KULeuven/Data/GFP KULeuven08] followed it. | ||
| + | * Paper found by LiFeng. | ||
| + | |||
| + | == Experiments == | ||
| + | [[Wetlab/SSrA/Experiment]] | ||
| + | |||
| + | |||
| + | == Other ideas == | ||
| + | Changing the degradation time by increasing the amount of Proteases? | ||
| + | * Proteases: | ||
| + | ** (ClpAP or ClpXP) and SSpB | ||
| + | ***I found only SspB at partregistry.org and there were [http://partsregistry.org/wiki/index.php/Part:BBa_K174000 BBa_K174000] which is sent and [http://partsregistry.org/wiki/index.php/Part:BBa_K174007 BBa_K174007] which is planning. | ||
| + | ***As for the part BBa_K174000 there is something with ClpXP | ||
| + | * Related papers not yet read: | ||
| + | ** [http://www.sciencemag.org/cgi/content/abstract/289/5488/2354 R1] | ||
| + | ** [http://www.jbc.org/content/283/36/24600.abstract R2] | ||
| + | **[http://www.nature.com/nsmb/journal/v10/n9/full/nsb0903-676.html R3] This one looks good to read. | ||
| + | * Find out if proteases have a negative effect on ''E. coli'' (like the protease HflB does) | ||
Revision as of 18:30, 27 October 2010
| Home | Project Overview | Speedy reporter | Speedy switch | Speedy protein degrader | Experiments and Parts | Applications | F.A.Q | About Us |
Contents |
Abstract
- We developed the fluorescent proteins with degradation tags which accelerate the reporter turnover. This provides better temporal resolution of gene expression profiling in single cells.
A central goal of synthetic biology is to explore the design principles that embedded in the architecture and operation of biological systems. To understand how biological systems are genetically assembled, synthetic biologists need tools to quantitatively describe the gene expression in model organisms. Fluorescent proteins, along with modern microscopes, allows real-time gene expression profiling at the level of individual living cells. Though the detection of fluorophores benefits from their stability, this advantage turns awkward when we are monitoring the dynamic cellular events which required measurements at higher temporal resolution. One approach to achieve such demand is specific and accelerated reporter degradation. Thus, we focus on the design of fusion proteins with degradation tags. Previous studies suggest the LVA tag has hitherto been most efficient tag to shorten the half-life of tagged protein in Escherichia coli. We added LVA tags to the C-terminal of different fluorescent proteins. Our results indicate that the half-life of fluorophores are significantly reduced. Therefore, we improved the temporal resolution of reporter proteins. This provides a promising tool for studying transient gene expression in the future.
Motivation
- How can we improve the temporal sampling of biological assay? We applied destabilized fluorescent proteins to achieve this goal.
Fluorescent proteins, for example, green fluorescent protein (GFP), are now widely used to visualize a protein’s distribution and dynamics in a subcellular compartment. The remarkable feature of the fluorescent proteins (FP) is that the fluorophore forms spontaneously (Chalfie et al., 1994) without the need of any substrate or cofactor (Heim et al., 1994). In addition, FP variants can be fused to virtually any protein of interest; thus, they can be used in many species for detection purposes within single living cells. FPs, together with modern microscopes, has become essential tools for studying spatial and temporal dynamics of cellular processes at high resolution.
The biophysical characteristics of fluorescent probes provide opportunities and, set limitations for fusion-protein studies. The fluorophore of GFP and its variants is contained within a barrel of beta-sheet protein (Ormo et al., 1996). This compact structure renders FPs with high photostability under a variety of conditions, even under treatment with protease (Chalfie et al., 1994). Once stable FPs formed, they are cleared from the cells in tens of hours (Andersen et al., 1998). This property allows prolonged imaging of cells, and easier detection when FPs accumulated. However, the stability of FPs limits its application in some studies which needs fast reporter turnover, for example, studies of transient gene expression. This also impedes the measurements of temporal expression pattern and behavior of proteins, because proteins at different stages of their lifetime are being detected. To overcome this limitation, we tried to develop destablized FPs.
There are several ways to generate FP variants with different spectral and expression properties. One approach is mutagenesis studies. Though this approach is fruitful (Delagrave et al., 1995; Heim et al., 1995; Zacharias et al., 2002), it seems impractical to screen possible mutations of each FP in ever-increasing FP libraries. Alternatively, we planed to control proteolysis by adding degradation tags to FPs (Andersen et al., 1998). This approach can be easily generalized to several FPs; thus, it provides extensibility to future applications.
Design: Controlled protein degradation in bacteria
- We added the degradation tag to each fluorescent protein. This approach effectively accelerates reporter turnover.
The flow of genetic information can—and sometimes need to — depend not only on its controlled synthesis but also equally on its controlled degradation. Indeed, protein degradation in natural systems is essential for cellular functions. Previous study showed that 2.7% of proteins are degraded per generation in Escherichia coli (E. coli.) growing in logarithmic phase (Fox and Brown, 1961). Control of protein turnover is required for cell cycle progression, signal transduction, and rapid responses to environmental challenges (Grunenfelder et al., 2001; Hengge-Aronis, 2002; Neher et al., 2003). Therefore, regulated degradation is expected to be critical for the development of synthetic circuits.
To control the half-life of specific proteins, we utilized the regulated proteolysis machineries naturally employed to bacterial systems. Regulated degradation is ubiquitous for prokaryotic and eukaryotic cells (Hershko and Ciechanover, 1998; Jenal and Hengge-Aronis, 2003), because they need to clear damaged or aberrant proteins while ignoring functional ones. A common strategy for substrate selection is adding degradation tags to target proteins. Then, certain proteases execute energy dependent degradation of those proteins. One prominent type of degradation tag in prokaryotes is the ssrA tag, a tmRNA encoded by ssrA gene. This tagging system is engaged in protein quality control throughout all eubacteria (Karzai et al., 2000). Though it was first described as a mechanism to clear obstructed ribosomes (Keiler et al., 1996), recent studies suggested ssrA-mediated tagging also plays a regulatory role in the expression of lac operon (Abo et al., 2000). This motivated us to control the protein expression via manipulating controlled proteolysis.
SsrA tag
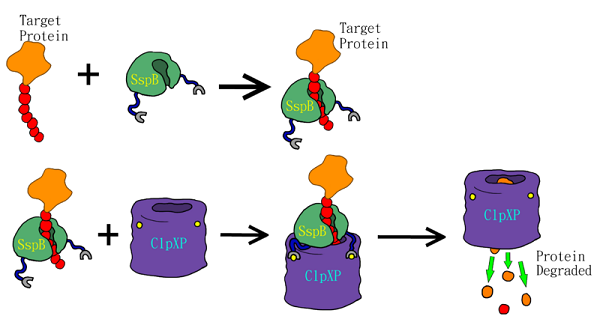
SsrA tag, encoded by the ssrA RNA, is important for protein quality control in bacteria. SsrA RNA is recognized as tmRNA because it has characteristics of both tRNA and mRNA (Atkins and Gesteland, 1996; Keiler et al., 1996). When E. coli. ribosomes stall during translation due to absence of a proper stop codon, ssrA ribosome-rescue system mediates C-terminal modification by adding the sequence AANDENYALAA to the the incomplete peptide (Keiler et al., 1996). SsrA tagging frees these ribosomes for other mRNAs, and targets the defective polypeptides for degradation by ClpXP and other ATP-dependent proteases (Gottesman et al., 1998). Previous studies suggest that 0.5% of protein products in E. coli.. receive ssrA tags (Lies and Maurizi, 2008). Bacterial strains lacking functional ssrA gene show slower growth (Oh and Apirion, 1991), reduction in motility (Komine et al., 1994), and subsided pathogenesis (Julio et al., 2000). These results indicate that ssrA tagging system play a major role in bacterial physiology.
The SsrA tag contains binding sites for the SspB adaptor and ClpXP protease (Levchenko et al., 2000). The SspB adaptor enhances substrate delivery by tethering SsrA-tagged proteins to the ClpX, which unfolds the tagged protein, and then translocates the denatured polypeptide into ClpP for degradation (Fig.1)(Sauer et al., 2004). The binding affinity of the ssrA tag to the SspB and ClpX plays key role in modulating substrate choice (Flynn et al., 2004) and the efficiency of degradation (Hersch et al., 2004; McGinness et al., 2006). One of the mutants, called LVA tag, further accelerates the degradation of tagged proteins compared to the wild-type LAA tag (Andersen et al., 1998). LVA tag has been applied to construct a fast synthetic gene oscillator, because it decreases protein lifetime and increases temporal resolution (Stricker et al., 2008). Thus, we added the LVA tag to FPs to maximize the temporal resolution of these reporters.
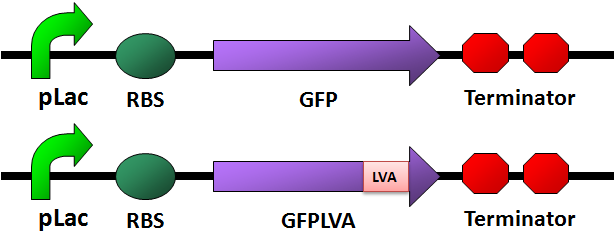
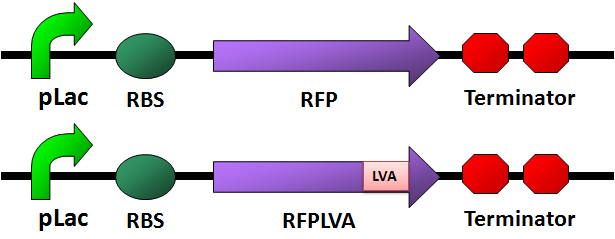
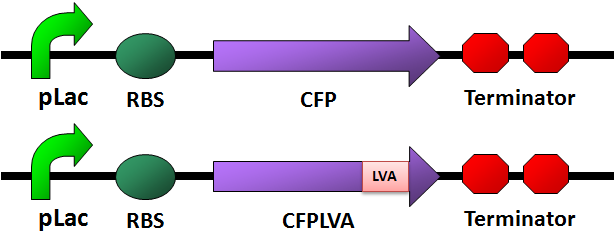
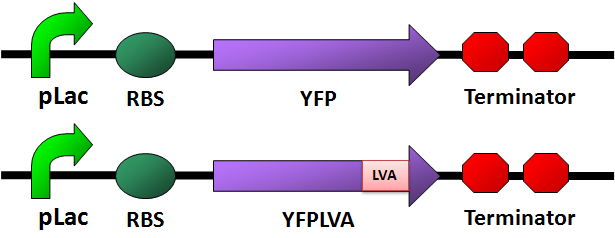
Circuit Design
- We design four fluorescence protein circuits. In each group, one is conrol, and another one is with LVA tag.
Measurement
Data analysis
Experiment overview
What are we doing?
Reference
- Abo, T., Inada, T., Ogawa, K., and Aiba, H. (2000). SsrA-mediated tagging and proteolysis of LacI and its role in the regulation of lac operon. EMBO J 19, 3762-3769.
- Andersen, J.B., Sternberg, C., Poulsen, L.K., Bjorn, S.P., Givskov, M., and Molin, S. (1998). New unstable variants of green fluorescent protein for studies of transient gene expression in bacteria. Appl Environ Microbiol 64, 2240-2246.
- Atkins, J.F., and Gesteland, R.F. (1996). A case for trans translation. Nature 379, 769-771.
- Chalfie, M., Tu, Y., Euskirchen, G., Ward, W.W., and Prasher, D.C. (1994). Green fluorescent protein as a marker for gene expression. Science 263, 802-805.
- Delagrave, S., Hawtin, R.E., Silva, C.M., Yang, M.M., and Youvan, D.C. (1995). Red-shifted excitation mutants of the green fluorescent protein. Biotechnology (N Y) 13, 151-154.
- Flynn, J.M., Levchenko, I., Sauer, R.T., and Baker, T.A. (2004). Modulating substrate choice: the SspB adaptor delivers a regulator of the extracytoplasmic-stress response to the AAA+ protease ClpXP for degradation. Genes Dev 18, 2292-2301.
- Fox, G., and Brown, J.W. (1961). Protein degradation in Escherichia coli in the logarithmic phase of growth. Biochim Biophys Acta 46, 387-389.
- Gottesman, S., Roche, E., Zhou, Y., and Sauer, R.T. (1998). The ClpXP and ClpAP proteases degrade proteins with carboxy-terminal peptide tails added by the SsrA-tagging system. Genes Dev 12, 1338-1347.
- Grunenfelder, B., Rummel, G., Vohradsky, J., Roder, D., Langen, H., and Jenal, U. (2001). Proteomic analysis of the bacterial cell cycle. Proc Natl Acad Sci U S A 98, 4681-4686.
- Heim, R., Cubitt, A.B., and Tsien, R.Y. (1995). Improved green fluorescence. Nature 373, 663-664.
- Heim, R., Prasher, D.C., and Tsien, R.Y. (1994). Wavelength mutations and posttranslational autoxidation of green fluorescent protein. Proc Natl Acad Sci U S A 91, 12501-12504.
- Hengge-Aronis, R. (2002). Signal transduction and regulatory mechanisms involved in control of the sigma(S) (RpoS) subunit of RNA polymerase. Microbiol Mol Biol Rev 66, 373-395
- Hersch, G.L., Baker, T.A., and Sauer, R.T. (2004). SspB delivery of substrates for ClpXP proteolysis probed by the design of improved degradation tags. Proc Natl Acad Sci U S A 101, 12136-12141.
- Hershko, A., and Ciechanover, A. (1998). The ubiquitin system. Annu Rev Biochem 67, 425-479.
- Jenal, U., and Hengge-Aronis, R. (2003). Regulation by proteolysis in bacterial cells. Curr Opin Microbiol 6, 163-172.
- Julio, S.M., Heithoff, D.M., and Mahan, M.J. (2000). ssrA (tmRNA) plays a role in Salmonella enterica serovar Typhimurium pathogenesis. J Bacteriol 182, 1558-1563.
- Karzai, A.W., Roche, E.D., and Sauer, R.T. (2000). The SsrA-SmpB system for protein tagging, directed degradation and ribosome rescue. Nat Struct Biol 7, 449-455.
- Keiler, K.C., Waller, P.R., and Sauer, R.T. (1996). Role of a peptide tagging system in degradation of proteins synthesized from damaged messenger RNA. Science 271, 990-993.
- Komine, Y., Kitabatake, M., Yokogawa, T., Nishikawa, K., and Inokuchi, H. (1994). A tRNA-like structure is present in 10Sa RNA, a small stable RNA from Escherichia coli. Proc Natl Acad Sci U S A 91, 9223-9227.
- Levchenko, I., Seidel, M., Sauer, R.T., and Baker, T.A. (2000). A specificity-enhancing factor for the ClpXP degradation machine. Science 289, 2354-2356.
- Lies, M., and Maurizi, M.R. (2008). Turnover of endogenous SsrA-tagged proteins mediated by ATP-dependent proteases in Escherichia coli. J Biol Chem 283, 22918-22929.
- McGinness, K.E., Baker, T.A., and Sauer, R.T. (2006). Engineering controllable protein degradation. Mol Cell 22, 701-707.
- Neher, S.B., Flynn, J.M., Sauer, R.T., and Baker, T.A. (2003). Latent ClpX-recognition signals ensure LexA destruction after DNA damage. Genes Dev 17, 1084-1089.
- Oh, B.K., and Apirion, D. (1991). 10Sa RNA, a small stable RNA of Escherichia coli, is functional. Mol Gen Genet 229, 52-56.
- Ormo, M., Cubitt, A.B., Kallio, K., Gross, L.A., Tsien, R.Y., and Remington, S.J. (1996). Crystal structure of the Aequorea victoria green fluorescent protein. Science 273, 1392-1395.
- Sauer, R.T., Bolon, D.N., Burton, B.M., Burton, R.E., Flynn, J.M., Grant, R.A., Hersch, G.L., Joshi, S.A., Kenniston, J.A., Levchenko, I., et al. (2004). Sculpting the proteome with AAA(+) proteases and disassembly machines. Cell 119, 9-18.
- Stricker, J., Cookson, S., Bennett, M.R., Mather, W.H., Tsimring, L.S., and Hasty, J. (2008). A fast, robust and tunable synthetic gene oscillator. Nature 456, 516-519.
- Zacharias, D.A., Violin, J.D., Newton, A.C., and Tsien, R.Y. (2002). Partitioning of lipid-modified monomeric GFPs into membrane microdomains of live cells. Science 296, 913-916.
Sequences
Project overview
A major goal of synthetic biology is to design and build custom signaling circuits, which can monitor internal and external states, with available molecular tools. To achieve a dynamic response and a quick adaptation to signals, we try to control the half-life of proteins by proteolysis.
SsrA tags
SSrA degradation tags to use:
| Tag | Amino Acid | Sequence |
| [ LVA] | RPAANDENYALVA | gctgcaaacgacgaaaactacgctttagtagct |
| [http://partsregistry.org/wiki/index.php/Part:BBa_M0050 LAA] | AANDENYALAA | gctgctaacgacgaaaactacgctctggctgct |
There were Biobricks for these two tags. BBa_M0040 for the LVA tag and BBa_M0042 for the LAA tag, but they are all "planning".
So I am wondering that if we can design or make this two parts for the iGEM?
Fluorescent proteins
| Effect | Class |
|---|---|
| Primers: (changes overline/underline and background) | |
| Forward Primer | FP |
| Overlapping Primer | OP |
| Reverse Primer | RP |
| Parts: (changes text color) | |
| Promoter | P |
| Ribosome Binding site | R |
| RNA | rna |
| Coding region | C |
| Terminator | T |
| Special | S |
| Cutting sites: (changes background) | |
| Biobrick cutting site | cut |
| Biobrick cutting site mutation | cutmut |
Fluorescent proteins to add degradation tags onto.
- [http://openwetware.org/wiki/IGEM:IMPERIAL/2007/Projects/Reporters#bibkey_Andersen FP comparison]
- Maturation time is the time until the protein starts shining.
GFP
- Used [http://partsregistry.org/wiki/index.php/Part:BBa_E0240 BBa_E0240] as a template:
| >[http://partsregistry.org/Part:BBa_E0240 BBa_E0240] | 876bp |
BBa_E0240+LVA (note LVA was added before the taataa double-stop codon)
| >BBa_E0240+LVA | 909bp |
Primers designed by Jesse in iGEM2008.
| >BBa_E0240+LVA | 909bp |
Primers: (directly copied from iGEM2008 wiki.) Outer primers: gaattcgcggccgcttctagag tcacacaggaaagtactagatgc (prefix + anneal: len:23, tm:56, %gc:43) ctgcagcggccgctactagta ataaacgcagaaaggccca (suffix + anneal: len:19, tm:56, %gc:47) Inner primers: Forward: CGACGAAAACTACGCTTTAGTAGCT taataatactagagccaggcatca (insert + anneal: len:24, tm:55, %gc38) Reverse: AAGCGTAGTTTTCGTCGTTTGCAGC tttgtatagttcatccatgccatg (insert + anneal: len:24, tm:55, %gc:38) Complimented part: CGACGAAAACTACGCTT (len:17, tm:50, %gc:47)
Final product is a Linear PCR DNA containing E0240+LVA.
Redesign Outer FP to include B0034 instead of B0032 (Lets save a cloning cycle)
XbaI+B0034+scar+E0040 start gcttctagag aaagaggagaaa tactag atgcgtaaaggagaagaacttttc (55C,24bp,38%GC) Total:52bp
RFP
PCR of [http://partsregistry.org/Part:BBa_I13507 BBa_I13507]
| >[http://partsregistry.org/Part:BBa_I13507 BBa_I13507] | 861bp |
| >[http://partsregistry.org/Part:BBa_I13507 BBa_I13507]+LVA | 869bp |
| >[http://partsregistry.org/Part:BBa_I13507 BBa_I13507]+LVA | 869bp |
Primers to order: Outer FP: gaattcgcggccgcttctagag aaagaggagaaatactagatggct (55C,24bp,38%GC) Total: Inner RP: CTACTAAAGCGTAGTTTTCGTCGTTTGCAGCagcaccggtggagtga (56C,16bp,63%GC) Total:47bp Primers that are the same as GFP: Inner FP: CGACGAAAACTACGCTTTAGTAGCTtaataatactagagccaggcatca (55C,24bp,38%GC) Total:49bp Outer RP: ctgcagcggccgctactagta tataaacgcagaaaggccca (56C,20bp,45%GC) Total:41bp Overlapping region: 55C,23bp,43%GC
YFP
- [http://partsregistry.org/wiki/index.php/Part:BBa_E0032 YFP with LVA tag]
CFP
- [http://partsregistry.org/wiki/index.php/Part:BBa_E0022 CFP with LVA tag]
- "[http://www.nature.com/nsmb/journal/v10/n9/full/nsb0903-676.html A tag and enhances its recognition by ClpXP.]" (Jabri03, Nature)
- [http://biocyc.org/ECOLI/NEW-IMAGE?type=ENZYME&object=CPLX0-3107 ClpXP degrades a whole range of proteins]. Does this mean that overexpression will kill E. coli due to it killing a lot more proteins than it suppose to??
- [http://www.cell.com/molecular-cell/retrieve/pii/S1097276504005908 A protein that has been covalently linked to SspB becomes a ClpXP substrate even in the absence of an SsrA tag]. (Bolon04, Cell)
- Does this mean that overexpression of SspB will kill E. coli due to it binding on different proteins?
ClpX
ClpP
SspB
- [http://biocyc.org/ECOLI/NEW-IMAGE?type=GENE&object=EG10978 Ecocyc] link.
- [http://biocyc.org/ECOLI/sequence-rc?type=GENE&object=EG10978 SspB] sequence.
| >SspB | 498bp |
FP: gcttctag atgGATTTGTCACAGCTAACAC temp:55C, len:22bp, %GC:41% (total 30bp) RP: ctgcagcggccgctactagta ttaCTTCACAACGCGTAATGC temp:55C, len:21bp, %GC:43% (total 41bp)
Measurements
- [http://www.ncbi.nlm.nih.gov/pmc/articles/PMC106306/?tool=pubmed one to follow] unless there's a better one KULeuven08 followed it.
- Paper found by LiFeng.
Experiments
Other ideas
Changing the degradation time by increasing the amount of Proteases?
- Proteases:
- (ClpAP or ClpXP) and SSpB
- I found only SspB at partregistry.org and there were [http://partsregistry.org/wiki/index.php/Part:BBa_K174000 BBa_K174000] which is sent and [http://partsregistry.org/wiki/index.php/Part:BBa_K174007 BBa_K174007] which is planning.
- As for the part BBa_K174000 there is something with ClpXP
- (ClpAP or ClpXP) and SSpB
- Related papers not yet read:
- [http://www.sciencemag.org/cgi/content/abstract/289/5488/2354 R1]
- [http://www.jbc.org/content/283/36/24600.abstract R2]
- [http://www.nature.com/nsmb/journal/v10/n9/full/nsb0903-676.html R3] This one looks good to read.
- Find out if proteases have a negative effect on E. coli (like the protease HflB does)
 "
"