Team:Mexico-UNAM-CINVESTAV/Protocols
From 2010.igem.org
(Difference between revisions)
| Line 17: | Line 17: | ||
#Digest 2-5ul, adding 1µl preboiled 10mg/ml RNAse A (see Reagents for preparation). | #Digest 2-5ul, adding 1µl preboiled 10mg/ml RNAse A (see Reagents for preparation). | ||
| - | |||
| - | |||
| - | + | ::KOAc solution: | |
| - | - 28.5 ml DDW | + | ::- 60 ml 5M potassium acetate |
| + | |||
| + | ::- 11.5 ml glacial acetic acid | ||
| + | |||
| + | ::- 28.5 ml DDW | ||
| Line 47: | Line 49: | ||
#Take competent E.coli cells from –80oC freezer. | #Take competent E.coli cells from –80oC freezer. | ||
| - | a. Use DH5α cells in most cases. | + | ::a. Use DH5α cells in most cases. |
| - | b. If want to cut at XbaI or other DAM- enzyme site, use SCS110 cells which are deficient in Dam and Dcm methylases. | + | ::b. If want to cut at XbaI or other DAM- enzyme site, use SCS110 cells which are deficient in Dam and Dcm methylases. |
#Turn on water bath to 42οC. | #Turn on water bath to 42οC. | ||
| - | #Put competent cells in a 1.5 ml tube (Eppendorf or similar). For transforming a | + | #Put competent cells in a 1.5 ml tube (Eppendorf or similar). For transforming a DNA construct, use 50 ul of competent cells. For transforming a ligation, use 100ul of competent cells. You may need more or less cells, depending how competent they are. |
| - | DNA construct, use 50 ul of competent cells. For transforming a ligation, use | + | |
| - | + | ||
| - | they are. | + | |
#Keep tubes on ice. | #Keep tubes on ice. | ||
| - | #Add 50 ng of circular DNA into E.coli cells. Incubate on ice for 10 min. to thaw | + | #Add 50 ng of circular DNA into E.coli cells. Incubate on ice for 10 min. to thaw competent cells. |
| - | competent cells. | + | |
#Put tube(s) with DNA and E.coli into water bath at 42οC for 45 seconds. | #Put tube(s) with DNA and E.coli into water bath at 42οC for 45 seconds. | ||
#Put tubes back on ice for 2 minutes to reduce damage to the E.coli cells. | #Put tubes back on ice for 2 minutes to reduce damage to the E.coli cells. | ||
| - | #Add 1 ml of LB (with no antibiotic added). Incubate tubes for 1 hour at 37 οC. | + | #Add 1 ml of LB (with no antibiotic added). Incubate tubes for 1 hour at 37 οC.(Can incubate tubes for 30 minutes, unless trying to grow DNA for ligation which)is more sensitive. For ligation, leave tubes for 1 hour.) |
| - | (Can incubate tubes for 30 minutes, unless trying to grow DNA for ligation which | + | #Spread about 100 ul of the resulting culture on LB plates (with appropriate antibiotic added – usually Ampicillin or Kanamycin.) Grow overnight. |
| - | is more sensitive. For ligation, leave tubes for 1 hour.) | + | |
| - | #Spread about 100 ul of the resulting culture on LB plates (with appropriate | + | |
| - | antibiotic added – usually Ampicillin or Kanamycin.) Grow overnight. | + | |
#Pick colonies about 12-16 hours later. | #Pick colonies about 12-16 hours later. | ||
| + | |||
| + | |||
| + | '''Double ligation''' | ||
Revision as of 07:48, 27 October 2010
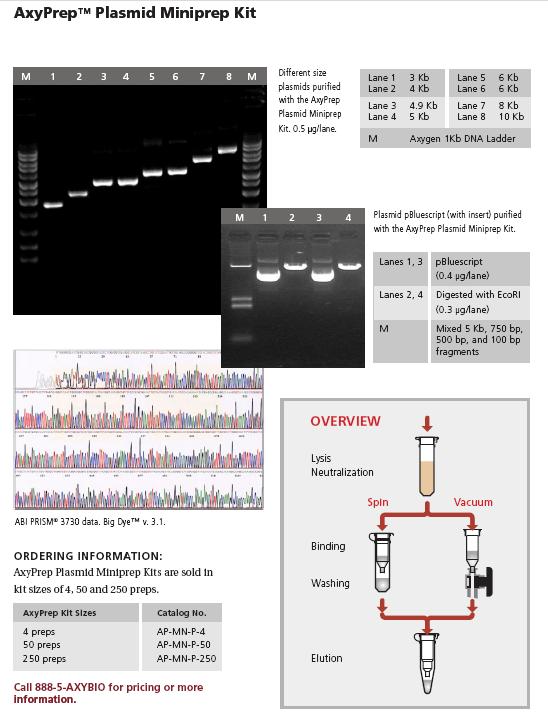
Alkaline Lysis Mini Plasmid Preps
- Grow O/N in 1.5 ml LMM or Terrific broth (see Reagents) with 75µg/ml Amp
- Pour into ependorf tube and spin down cells at 7-8K for 2 min
- Aspirate s/n and resuspend in 50µl 25mM Tris pH 8, 10 mM EDTA; leave lids open
- Add 100µl of freshly prepared 1% SDS, 0.2M NaOH (5ml = 100ul 10M NaOH added to 4.4ml DDW then 500µl 10% SDS). Add it forcefully and you don't need to vortex
- Add 75µl KoAc solution and vortex
- Add 100µl of phenol/CHI3, close lids, vortex
- Spin 13K for 2 mins
- Remove supernatant, add to 500µl ethanol. Vortex and spin at 13 K for 5 min
- Aspirate s/n, removing all ethanol
- Resuspend in 50µl TE
- Digest 2-5ul, adding 1µl preboiled 10mg/ml RNAse A (see Reagents for preparation).
- KOAc solution:
- - 60 ml 5M potassium acetate
- - 11.5 ml glacial acetic acid
- - 28.5 ml DDW
DNA Isolation for Low-Melting Point Agarose
(using elu-tip method)
- Excise fragment from gel and estimate volume.
- Add 1/100 volume of 1 M Tris pH 7.5, 1/50 volume of 0.5 M EDTA, and 1/100 volume of 5M NaCl.
- Incubate at 68°C for 10 minutes.
- Vortex and remove to a small tube.
- Incubate at 37°C for 5 minutes.
- Phenol extract 2-3 times (phenol at 42°C, spin at 13 K for 2 minutes.)
- Ether extract 1 time. Place tubes at 65°C, then speed-vac 2 to 5 minutes. Repeat procedure about 4 times to get rid of residual ether.
- Ethanol precipitate DNA and suspend pellet in 1 ml of low salt buffer (from elu-tip column protocol)
- Following the elu-tip protocol booklet, wash the column by pushing 5 ml of low salt buffer through the matrix at a rate of 0.5-1.0 ml/minute. The column may be incubated in the low salt buffer ³ 2 hours to improve recovery.
- Load DNA sample onto the column slowly (1-2 drops/second). NOTE: When recovering DNA from low-melt temperature agarose, use of the pre-filter is not recommended. Consult the protocols booklet for specific parameters of different types of nucleic acid purification (i.e. DNA purification when LMP agarose isn't used).
- Wash the column with 2-3 mls of pre-warmed (42°C) low salt buffer.
- Elude DNA with 0.4 ml of high salt buffer. Do a total of 2-3 washes as desired to be certain of good recovery.
- Ethanol precipitate DNA and resuspend in TE or dH2O.
Transformation Protocol Using Heat Shock
- Take competent E.coli cells from –80oC freezer.
- a. Use DH5α cells in most cases.
- b. If want to cut at XbaI or other DAM- enzyme site, use SCS110 cells which are deficient in Dam and Dcm methylases.
- Turn on water bath to 42οC.
- Put competent cells in a 1.5 ml tube (Eppendorf or similar). For transforming a DNA construct, use 50 ul of competent cells. For transforming a ligation, use 100ul of competent cells. You may need more or less cells, depending how competent they are.
- Keep tubes on ice.
- Add 50 ng of circular DNA into E.coli cells. Incubate on ice for 10 min. to thaw competent cells.
- Put tube(s) with DNA and E.coli into water bath at 42οC for 45 seconds.
- Put tubes back on ice for 2 minutes to reduce damage to the E.coli cells.
- Add 1 ml of LB (with no antibiotic added). Incubate tubes for 1 hour at 37 οC.(Can incubate tubes for 30 minutes, unless trying to grow DNA for ligation which)is more sensitive. For ligation, leave tubes for 1 hour.)
- Spread about 100 ul of the resulting culture on LB plates (with appropriate antibiotic added – usually Ampicillin or Kanamycin.) Grow overnight.
- Pick colonies about 12-16 hours later.
Double ligation
 "
"