Team:Stockholm/Project Idea/Proteins
From 2010.igem.org
(Difference between revisions)
m (→Human basic fibroblast growth factor, bFGF) |
|||
| Line 6: | Line 6: | ||
== Proteins == | == Proteins == | ||
| - | === Superoxide dismutase 1 | + | === Superoxide dismutase 1 (SOD1) === |
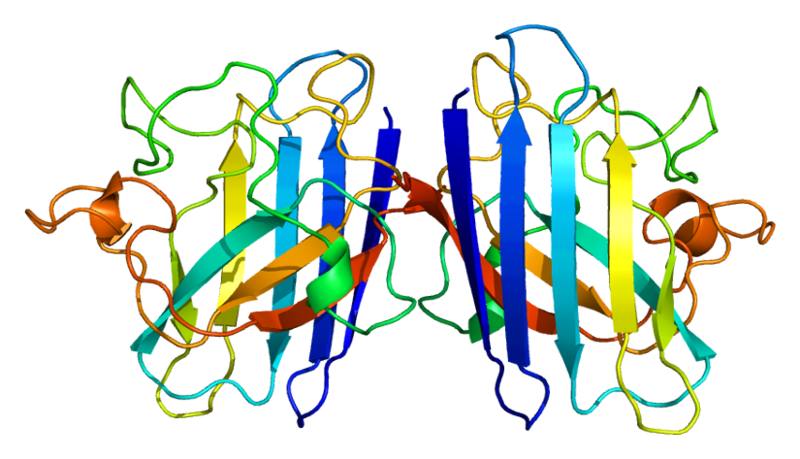
| - | [http:// | + | Human soluble Superoxide dismutase 1 (SOD1) is a soluble cytoplasmic protein functional as a homodimer that binds copper and zink ions. SOD1 catalyzes the reaction O<sup>-</sup><sub>2</sub> + O<sup>-</sup><sub>2</sub> + 2H<sup>+</sup> → H<sub>2</sub>O<sub>2</sub> + O<sub>2</sub>, protecting the cell from oxidative damage. SOD1 was first cloned and expressed in ''Escherichia coli'' by [http://www.ncbi.nlm.nih.gov/pubmed/3889846 Hallewell ''et al''., (1985)]. |
| - | + | {|border="1" align="center" cellpadding="3" cellspacing="0" | |
| - | + | !colspan="2"|Gene (cDNA) | |
| - | + | |rowspan="10" width="250"|[[Image:SOD1_dimeric.png|250px]]<br />3D structure of human SOD1 in its dimeric form. Primary citation [http://www.ncbi.nlm.nih.gov/pubmed/20822138 Leinartaite ''et al''. (2010)] | |
| - | [[Image:SOD1_dimeric.png| | + | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
|- | |- | ||
| - | | | + | |width="130"|'''Length''' |
| - | | 465 bp | + | |width="130"|465 bp |
|- | |- | ||
| - | | | + | |'''Edited nucleotide(s)''' |
| - | | | + | |nt331 A → G |
|- | |- | ||
| - | | | + | |'''Removed restr. site(s)''' |
| - | | | + | |PfeI |
|- | |- | ||
| - | | ''' | + | |'''GenBank''' |
| - | | | + | |[http://www.ncbi.nlm.nih.gov/nucleotide/38489879?report=genbank&log$=nucltop&blast_rank=22&RID=CAM83NYN01S AY450286.1]<br /> |
|- | |- | ||
| - | | | + | !colspan="2"|Protein |
| - | + | ||
|- | |- | ||
| - | | | + | |'''Length''' |
| - | | | + | |154 aa |
|- | |- | ||
| - | | Fasta | + | |'''Size''' |
| - | | [http://www.ncbi.nlm.nih.gov/protein/49456443?report=fasta SOD1] | + | |15,936 Da |
| + | |- | ||
| + | |'''Fasta''' | ||
| + | |[http://www.ncbi.nlm.nih.gov/protein/49456443?report=fasta SOD1]<br /> | ||
| + | |- | ||
| + | |colspan="2"|First reported by [http://www.ncbi.nlm.nih.gov/pubmed/3889846 Hallewell ''et al''., (1985)]. | ||
|} | |} | ||
| - | + | ---- | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | Yeast copper chaperon protein (yCCS) is a | + | === Yeast copper chaperon (yCCS) === |
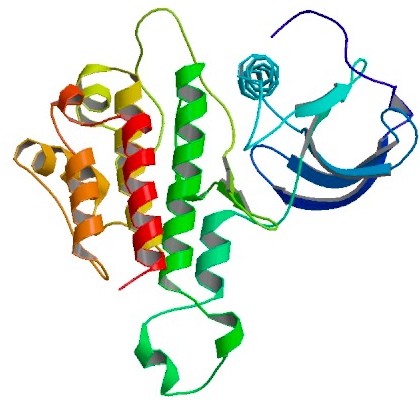
| + | Yeast copper chaperon protein (yCCS) is a helper chaperon specific for copper/zinc superoxide dismutase located to the cytoplasm. yCCS generates fully metallized, active SOD1 proteins that in turn protects the cell from oxidative damage. | ||
yCCS has been shown to successfully mediate the delivery of copper ions to human SOD1 ([http://www.ncbi.nlm.nih.gov/pubmed/15358352 Ahl ''et al''. 2003]). Co-expression of SOD1 and yCCS yields proteins with higher copper contents, leading to increased activity and more stable proteins. | yCCS has been shown to successfully mediate the delivery of copper ions to human SOD1 ([http://www.ncbi.nlm.nih.gov/pubmed/15358352 Ahl ''et al''. 2003]). Co-expression of SOD1 and yCCS yields proteins with higher copper contents, leading to increased activity and more stable proteins. | ||
| - | |||
| - | |||
| - | + | {|border="1" align="center" cellpadding="3" cellspacing="0" | |
| - | + | !colspan="2"|Gene (cDNA) | |
| - | {| | + | |rowspan="10" width="250"|[[Image:YSOD+yCCS_interaction.jpg|250px]]<br />3D structure of yCCS interacting with yeast superoxide dismutase (ySOD) in it's monomeric form. Ions indicated as gray orbs. Primary citation [http://www.ncbi.nlm.nih.gov/pubmed/11524675 Lamb ''et al''. 2001] |
| - | | | + | |
| - | | | + | |
|- | |- | ||
| - | | | + | |width="130"|'''Length''' |
| - | | | + | |width="130"|750 bp |
|- | |- | ||
| - | | | + | |'''Edited nucleotide(s)''' |
| - | | | + | |nt257 T → C |
|- | |- | ||
| - | | | + | |'''Removed restr. site(s)''' |
| - | | | + | |EcoRI |
|- | |- | ||
| - | | ''' | + | |'''GenBank''' |
| - | | | + | |[http://www.ncbi.nlm.nih.gov/nuccore/NM_001182535.1?report=genbank&log$=seqview NM_001182535.1]<br /> |
|- | |- | ||
| - | | | + | !colspan="2"|Protein |
| - | + | ||
|- | |- | ||
| - | | | + | |'''Length''' |
| - | | | + | |249 aa |
|- | |- | ||
| - | | Fasta | + | |'''Size''' |
| - | | [http://www.ncbi.nlm.nih.gov/protein/596088?report=fasta yCCS] | + | |27,330 Da |
| + | |- | ||
| + | |'''Fasta''' | ||
| + | |[http://www.ncbi.nlm.nih.gov/protein/596088?report=fasta yCCS]<br /> | ||
| + | |- | ||
| + | |colspan="2"|First reported by [http://www.ncbi.nlm.nih.gov/pubmed/9295278 Culotta ''et al''. (1997)].<br /> | ||
|} | |} | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| Line 102: | Line 82: | ||
| - | === Human basic fibroblast growth factor | + | === Human basic fibroblast growth factor (bFGF) === |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | {| | + | {|border="1" align="center" cellpadding="3" cellspacing="0" |
| - | | | + | !colspan="2"|Gene (cDNA) |
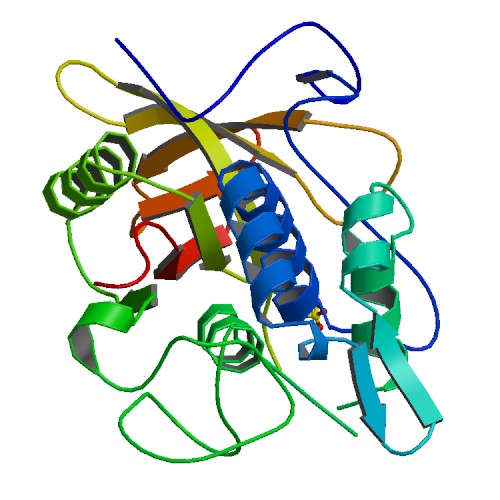
| - | | | + | |rowspan="10" width="250"|[[Image:BFGF.jpg|250px]]<br />3D structure of bFGF. Primary citation [http://www.ncbi.nlm.nih.gov/pubmed/20133753 Bae ''et al''. 2010] |
|- | |- | ||
| - | | | + | |width="130"|'''Length''' |
| - | | 468 bp | + | |width="130"|468 bp |
|- | |- | ||
| - | | | + | |'''Edited nucleotide(s)''' |
| - | | | + | |nt341 C → T |
|- | |- | ||
| - | | | + | |'''Removed restr. site(s)''' |
| - | | | + | |AgeI |
|- | |- | ||
| - | | ''' | + | |'''GenBank''' |
| - | | | + | | <br /> |
|- | |- | ||
| - | | | + | !colspan="2"|Protein |
| - | + | ||
|- | |- | ||
| - | | | + | |'''Length''' |
| - | | | + | |155 aa |
|- | |- | ||
| - | | Fasta | + | |'''Size''' |
| - | | [http://www.ncbi.nlm.nih.gov/protein/153285461?report=fasta bFGF] | + | |17,353 Da |
| + | |- | ||
| + | |'''Fasta''' | ||
| + | |[http://www.ncbi.nlm.nih.gov/protein/153285461?report=fasta bFGF]<br /> | ||
| + | |- | ||
| + | |colspan="2"|First reported by <unknown><br /> | ||
|} | |} | ||
| - | + | ---- | |
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
=== Protein A, z domain === | === Protein A, z domain === | ||
Revision as of 07:39, 27 October 2010
 |

|
 |

|
 |

|

|

|
 "
"