Team:Mexico-UNAM-CINVESTAV/Project/Our project
From 2010.igem.org
m (new our project) |
m (tools) |
||
| Line 1: | Line 1: | ||
| - | ---- | + | {{Mexico-UNAM-CINVESTAV-HEADER}} |
| + | {{Mexico-UNAM-CINVESTAV-COUNTDOWN}} | ||
'''PROJECT''' | '''PROJECT''' | ||
Revision as of 02:55, 26 October 2010
PROJECT
First we organize a cold regulation system at post-traductional level using a constitutive gene that E. coli has to cope with a sudden downshift (from higher temperature down to 15ºC up to 10ºC). Also we had added an antifreeze protein, in order to help the cell to prevent water from undergoing a freezing process creating strong hydrogen bounds and inducing a vitrification process rather than a normal crystalization process. In that way the cytoplasm and the cell membrane did not damage at freezing temperatures. This both strategies may create new biobricks that can function as a “cold kit” to both synthesize proteins and build an E. coli mutant that allow us to work with it at low temperatures. Even we are trying to overcome other human issues like the plants and crops maintenance, in order to survive the cold weather countries like Canada, USA, Northern European countries and of course the frosts occurring in the north of Mexico and high mountains of country. In order to improve efficiency to our cold system we build four constructions to prove the different kind of regulation that the mechanism can allow.
• Firstly we took the promoter with all the parts involved, from the promoter polymerase-binding zone, to the Downstream Box that is inside the protein DNA sequence.
• In a second construction we took all the regulation system except the promoter to define if another promoter different to the CspA can be regulated with the freeze system.

• We also have the CspA promoter alone so we can put it controlling another gene and see if this can function at low temperatures.
• For a final construction, we took the Cold Box, the UTR region and the Shine-Dalgarno sequence with the exception of the Downstream Box and the promoter since we are not sure if having two methionines at the beginning of the protein shall cause problems to the translational system in the ribosomes, even if it’s an important point of regulation in the freeze system. Despite we have founded in some reports that the freeze system could be express under these conditions, we are trying to confirm this and to valuate which construction is better.

To make these constructions we will use primers that allow us to make PCR and obtain the different pieces of the Cold Shock promoter. These primers have been designed with the specifics requirements, so they have segments of cutting restriction enzymes that the Biobrick Standard Assembly 10 request. This allow us to cut and paste anywhere we want as a biobrick; using this we also can do the constructions with the AFP that we want, and express the protein at low temperatures. To characterize the promoter we use the Relative Promoter Units (RPU). This consist in proving the biobricks with GFP and see their expresion compared to an stablished promotor that is already tested. With this we can asure that our promoter works fine or is not quite good, and we can. Our biobrick have been located in the pSB3K3 kanamycin vector with the E0240 GFP. The promoter I20260 have been located in the same vector with the same GFP than us (E0240) and then we compare both ones.
To send the biobricks we use the pSB1C3 chloramphenicol vector as the Biobrick Standard Assembly 10 request.
The diverse constructions of the promoter in biobrick format with the AFP have been settled in the pSb1A2 ampycilin vector to express and prove that the biobricks are working as we want. This is our final construction and is the one we are expecting to serve as an antifreeze system.
Machinery that regulates the expression + AFP = "E.coldi"
"http://upload.wikimedia.org/wikipedia/commons/0/08/Walschaerts_motion.gif"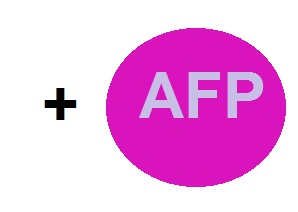

Baneyx, François and Mujacic, Mirna; (2002); Cold-Inducible Promoters for Heterologous Protein Expression; from Methods in Molecular Biology; Vol. 205 Humana Press Inc.[http://www.springerlink.com/content/qx66q8875x0q7043/#section=86530&page=1]
D’amico, Salvino, et al; (2006); Psychrophilic microorganisms: challenges for life; European Molecular Biology Organization Vol.7 No. 4 p 385-389[http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1456908/]
de Camargo, A. P. and de Camargo, M. B. P.; (2008); Frost in Coffee Crops: Frost Characteristics, Damaging Effects on Coffee and Alleviation Options, in Coffee: Growing, Processing, Sustainable Production: A Guidebook for Growers, Processors, Traders, and Researchers (ed J. N. Wintgens), Wiley-VCH Verlag GmbH, Weinheim, Germany. doi: 10.1002/9783527619627.ch11
Chattopadhyay M. K; (2007); Antifreeze Proteins of Bacteria; Resonance p 25-30[http://www.ias.ac.in/resonance/December2007/p25-30.pdf]
Davies, Peter L. and Hew, Choy L.; (1990); Biochemistry of fish antifreeze proteins; The FASEB journal; Vol. 4 p 2460-2468[http://www.fasebj.org/cgi/content/abstract/4/8/2460]
Heather A. Thieringer, Pamela G. Jones, and Masayori Inouye; (1998); Cold Shock and Adaptation; Bio Essays 20.1: 40-57[http://onlinelibrary.wiley.com/doi/10.1002/(SICI)1521-1878(199801)20:1%3C49::AID-BIES8%3E3.0.CO;2-N/abstract]
Horn G., et al.; (2007); Structure and function of bacterial cold shock proteins; Cellular and Molecular Life Sciences; Vol. 64 p. 1457 – 1470
Leslie Samuel B., et al; (1995); Trehalose and Sucrose Protect Both Membranes and Proteins in Intact Bacteria during Drying; Applied and Environmental Microbiology; Vol. 61, No. 10[http://www.ncbi.nlm.nih.gov/pmc/articles/PMC167656/pdf/613592.pdf]
Pankow J. F.; (1991); Aquatic Chemistry Concept; EUA CRC Press LLC. p.683[http://books.google.com.mx/books?hl=es&lr=&id=bV3BZVSw-lYC&oi=fnd&pg=PA3&dq=Aquatic+Chemistry+Concept&ots=o0GQl0B_Ng&sig=ncS8JTnfM3GEG1_hO-6SV2YqqwE]
Yamanaka, Kunitoshi; (1999); Cold Shock Response in Escherichia coli; Journal of Molecular and Microbiological Biotechnology 1(2): 193-202[http://www.horizonpress.com/jmmb/v1/v1n2/03.pdf]
 |
 |
 |
 |
 "
"





