Team:Groningen/19 July 2010
From 2010.igem.org
| Line 79: | Line 79: | ||
| - | We’ve transformed all the constructs ordered from Mr. Gene to a new batch of good competent cells and grew single colonies in liquid culture. These were miniprepped and also used to make glycerol stocks. | + | We’ve transformed all the constructs ordered from Mr. Gene to a new batch of good competent cells and grew single colonies in liquid culture. These were miniprepped and also used to make glycerol stocks. The plasmids were checked by restriction with EcoRI and PstI and had the right size. |
| + | |||
It was decided that we will also try to make the pNZ8048 plasmid biobrick compatible so that we can also try to express our chaplins in Lactococcus lactis using this nisin inducible plasmid. The same forward primer can be used to insert the biobricks and another reversed primer was ordered. | It was decided that we will also try to make the pNZ8048 plasmid biobrick compatible so that we can also try to express our chaplins in Lactococcus lactis using this nisin inducible plasmid. The same forward primer can be used to insert the biobricks and another reversed primer was ordered. | ||
Revision as of 19:23, 24 October 2010
Week 29
Modellers:
After lots of meetings the time has come to really start up the modelling part of iGEM. This week we thought about what to model and how. We have looked up and read lots of articles.
David
Drying biofilms
After formation of biofilm on the pieces of wood and ceramics, the material was taken out of the wells plates and put into empty petri dishes. This was put into the 37 C oven for 3 days. Inspection of these dried in biofilms was done by stereomicroscopy.
David & Peter
Dispersant effects chaplin proteins
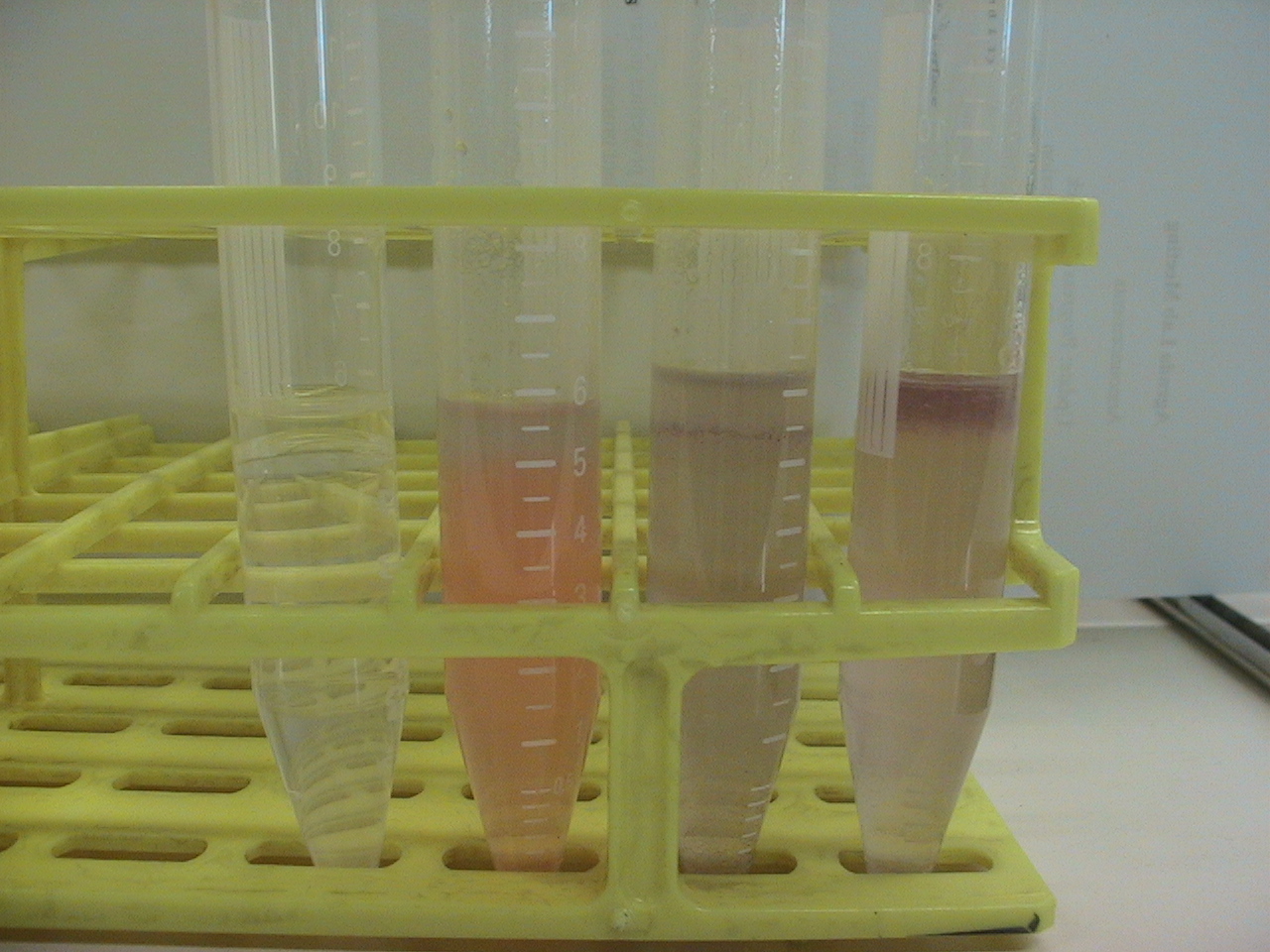
5 ml of demiwater and 0.5 ml olive oil, adding 500 microliter of 15 mg/ml monomeric chaplins to one tube, 0.5 ml vortexed assembled chaplins, and two controls without chpls added. Also ad 30 microliter congo red solution (3 mg/micro liter) to all samples exept one of the two controls. This congo red will stain the assembled chapl Then vortex for 1 min add max speed, then let the samples sit for 10 min. After this closeup pictures are taken of the oil water interface.
Before Shaking
Left to right: Water+oil, Water+oil+CongoRed+AssembledChaplins, Water+Oil+CongoRed+MonomericChaplins, Water+Oil+CongoRed
After Shaking:
Left to right: Water+oil, Water+oil+CongoRed, Water+Oil+CongoRed+MonomericChaplins, Water+Oil+CongoRed+AssembledChaplins
As aspected the monomeric chaplins helped to disperse the oil throughout the water, this is nicely visualized by the dispersion of the CongoRed throughout the tube. The assembled chaplins do not show this behavior and seem to have grouped within the oil, which is still on top of the water.
Close ups: Shortly after shaking, left: Control (H20 + Oil), right (Water + Oil + Monomeric Chaplins).
In close up, it is observed that the monemeric chaplins do indeed help to disperse the oil in the water. As one can see, small droplets of oil are abundant in the water + Chaplins, were no droplets are observed at the same timepoint after shaking in the control.
Arend Jan
We’ve transformed all the constructs ordered from Mr. Gene to a new batch of good competent cells and grew single colonies in liquid culture. These were miniprepped and also used to make glycerol stocks. The plasmids were checked by restriction with EcoRI and PstI and had the right size.
It was decided that we will also try to make the pNZ8048 plasmid biobrick compatible so that we can also try to express our chaplins in Lactococcus lactis using this nisin inducible plasmid. The same forward primer can be used to insert the biobricks and another reversed primer was ordered.
Component µl Final concentration Primer pNZ89bbs-for1 5 300nM Primer pNZ8048bbs-rev 5 300nM 5x Buffer HF 10 1x dNTP’s 2 200µM Template 1 ~1ng Phusion Pol. (Finnzymes)1/0.5 2/1u MQ 26/26.5 - 98°C, 3’ - 98°C, 30’’ 25X - 50°C, 30’’ - 72°C, 5’ - 72°C, 10’
 "
"