Team:Heidelberg/Notebook/miRNA Kit/September
From 2010.igem.org
(Difference between revisions)
Laura Nadine (Talk | contribs)
(New page: ==02/09/2010== * cloning of different shRNAs into pcDNA5/FRT/TO * quick and dirty cloning of tuning and tetR construct goes on ==03/09/2010== * ... and on ==04/09/2010== * mini-prep of p...)
Newer edit →
(New page: ==02/09/2010== * cloning of different shRNAs into pcDNA5/FRT/TO * quick and dirty cloning of tuning and tetR construct goes on ==03/09/2010== * ... and on ==04/09/2010== * mini-prep of p...)
Newer edit →
Revision as of 02:53, 24 October 2010
02/09/2010
- cloning of different shRNAs into pcDNA5/FRT/TO
- quick and dirty cloning of tuning and tetR construct goes on
03/09/2010
- ... and on
04/09/2010
- mini-prep of pcDNA5 with shRNAs 1, 2, 3, 5, 5* and 6 (each four colonies from two independent transformations)
- PCR of shRNA constructs (including shRNA 7, 8 and 9 as well)
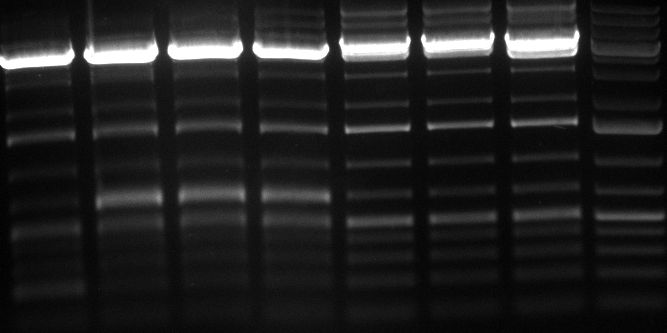
- gel: fine, everywhere amplified sequences below 200 bp
- digestion of promising tuning constructs with BspEI and StuI
- gel: plasmids seem to be cut only once again (problem maybe BspEI), size is correct
- suggestion: another digestion on monday with SalI and KpnI, then: submission for sequencing
- gel: plasmids seem to be cut only once again (problem maybe BspEI), size is correct
06/09/2010
- digestion of tuning construct with KpnI and SalI result in weird band pattern on the gel
- PCR and purification of the binding sites looked fine on the gel
- submission of tuning constructs and pcDNA5 containing different miRNAs for sequencing
07/09/2010
- repetition of TetR and tuning construct cloning
- purification and digestion of PCR amplified binding sites with NotI
08/09/2010
- cloning of shRNAs in pcDNA5 again
- further digestion of binding sites with AscSI (analog to SgfI) and purification
- preparation of psyCHECK2 for insertion of binding sites and shRNAs for first test measurements
- transformation of TetR constructs
- ligation of both inserts for the tuning construct, extraction of the right insert (3000bp) and ligation into the backbone
09/09/2010
- colony PCR of TetR constructs are positive
- inoculate TetR for miniprep
10/09/2010
- miniprep of TetR
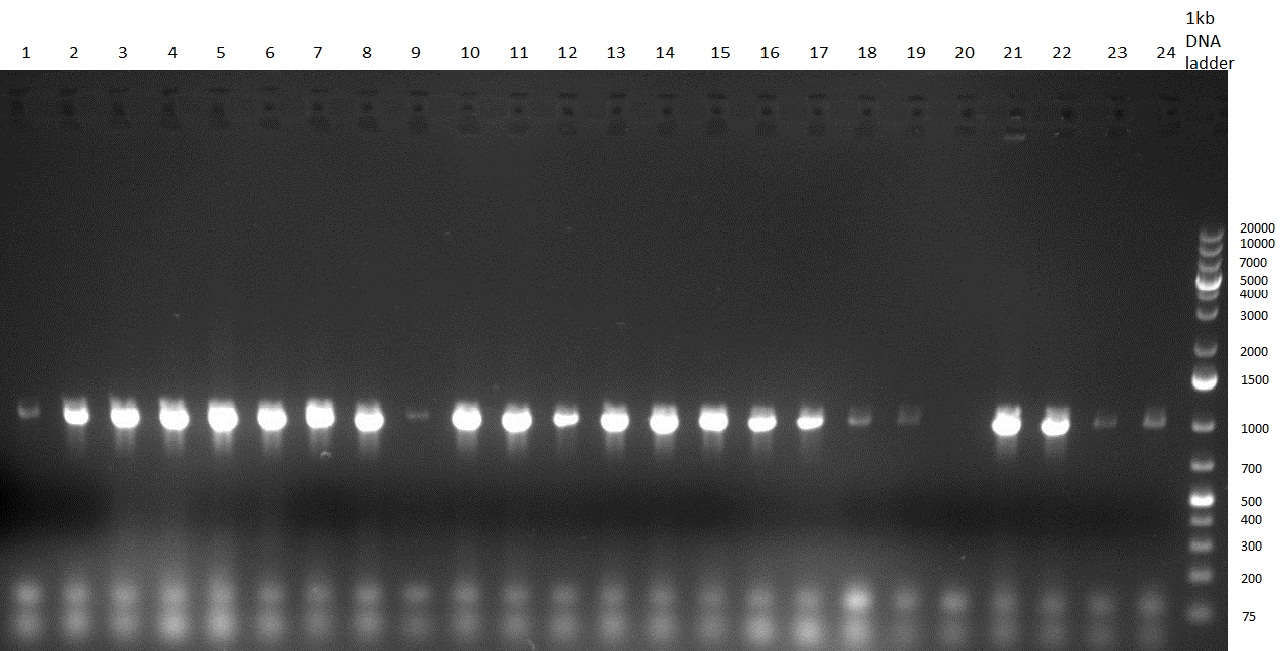
- test digest of TetR with BspEI & NotI and with SgfI gave promising results
- YES it was done with marker instead of loading dye but:
- for lanes 2-4 you see a stronger band at 700 pb (size of TetR)
- for landes 5-7 you see a stronger band at 5000 bp as it was linearized with SgfI
- YES it was done with marker instead of loading dye but:
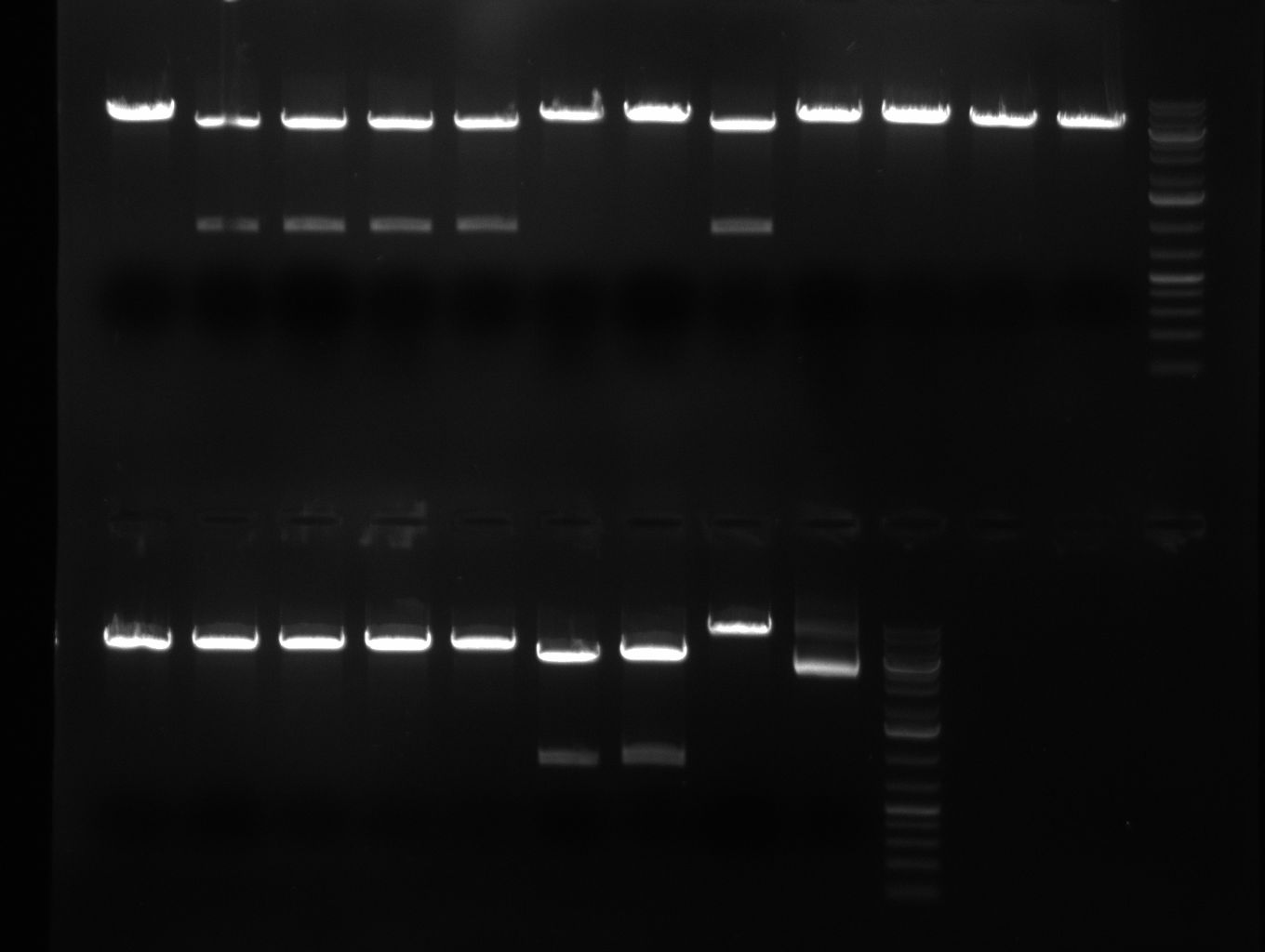
- colony PCR of binding site in PsiCheck and tuning construct.. tuning construct looks promising, BS nothing on the gel except for primers
- colony PCR of shRNAs look promising even though we cannot say whether still the old shRNA is inside
- PCR of CMV shRNA which leads to a size of 1200bp
11/09/2010
- miniprep of tuning construct and assorted shRNA BS PsiCheck colonies. Repetition of test PCR (for tuning primer L11 and L13, for BS primer L1 and 86a (dominik)) with miniprep template. Tuning construct looks kind of good on the gel, but I don't want to get you hopes up too soon.. it will be send for sequencing on monday.
- Tet Repressor cloned!!!
- shRNA 2,3,5,7,8 and 9 seems to be cloned according to test digest

|
|
|||
 "
"