Team:UCL London/Bioprocess Flowsheet Development
From 2010.igem.org
| Line 10: | Line 10: | ||
E. coli is an established production system of choice for antibody fragments used in therapeutic applications. One reason is that E. coli provides the means to progress from antibody selection to Good Manufacturing Practice (GMP) production of antibodies in a rapid manner. The other reason is the fact that high production levels of antibody fragments are attainable when using E. coli. As of 2004, there have been several Fabs or Fab’s in clinical trials run by Genentech and Celltech, where the fragments have been produced using E. coli (Anderson et al., 2004). | E. coli is an established production system of choice for antibody fragments used in therapeutic applications. One reason is that E. coli provides the means to progress from antibody selection to Good Manufacturing Practice (GMP) production of antibodies in a rapid manner. The other reason is the fact that high production levels of antibody fragments are attainable when using E. coli. As of 2004, there have been several Fabs or Fab’s in clinical trials run by Genentech and Celltech, where the fragments have been produced using E. coli (Anderson et al., 2004). | ||
| + | |||
'''Fermentative Pathways''' | '''Fermentative Pathways''' | ||
Revision as of 22:57, 22 October 2010
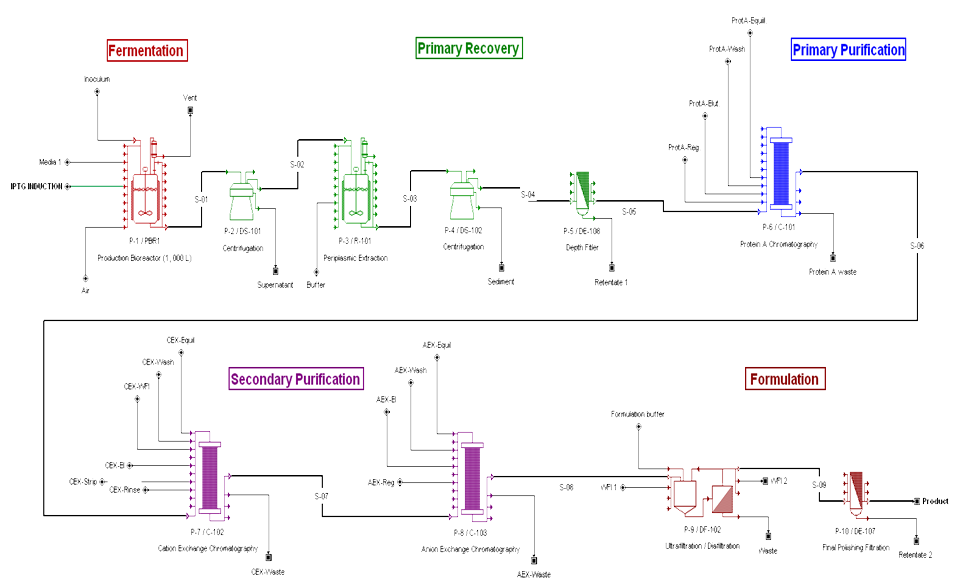
Bioprocess Flowsheet
According to Makrides (1996) the periplasm of E.coli contains only about 4% of the total cell protein. The periplasmic expression allows expression of fragments to be effectively concentratd. Other reports (Humphreys, 2004) that the antibody expression of Fab fragments can take place in the periplasm of E.coli and purified with an aqueous periplasmic heat extraction, which eliminates most of the host cytoplasmic and membrane proteins, followed by ion exchange chromatography.
E. coli is an established production system of choice for antibody fragments used in therapeutic applications. One reason is that E. coli provides the means to progress from antibody selection to Good Manufacturing Practice (GMP) production of antibodies in a rapid manner. The other reason is the fact that high production levels of antibody fragments are attainable when using E. coli. As of 2004, there have been several Fabs or Fab’s in clinical trials run by Genentech and Celltech, where the fragments have been produced using E. coli (Anderson et al., 2004).
Fermentative Pathways
Host: Escherichia Coli
Advantages
Provides a wide choice of cloning vectors
Easily controlled gene expression
Gives large yields
Secretes good protein
Provides fast growth rate
Disadvantages
Lacks post-translational modifications
Posses high levels of endotoxins
Forms inclusion bodies (i.e. protein aggregates)
Escherichia coli produces antibody fragments rather than whole antibodies due to the fact that it lacks post-translational modifications and also since polymeric polypeptide assembly is not well supported (Johansson, 2007)


 "
"


 Twitter
Twitter Facebook
Facebook UCL
UCL Flickr
Flickr YouTube
YouTube