Team:Freiburg Bioware/NoteBook/Labjournal/October
From 2010.igem.org
(→Assembly of pSB1C3_CMV_DARPin_VP2/3_insCap and pSB1C3_CMV_DARPin_VP2/3_HSPG-KO) |
(→Assembly of pSB1C3_CMV_DARPin_VP2/3_insCap and pSB1C3_CMV_DARPin_VP2/3_HSPG-KO) |
||
| Line 667: | Line 667: | ||
|~ 475 bp | |~ 475 bp | ||
|-- | |-- | ||
| - | + | <br/> | |
| - | + | <br/> | |
| + | [[Image:Freiburg10 4 10 Dig.png]] | ||
Revision as of 18:47, 4 October 2010
- March (labday 1)
- April (labday 2 - 5)
- May (labday 6 - 17)
- June (labday 18 - 45)
- July (labday 46 - 75)
- August part 1 (labday 76 - 92)
- August part 2 (labday 93 - 106)
- September part 1 (labday 107 - 123)
- September part 2 (labday 124 - 135)
- October part 1 (labday 136 - 145 )
- October part 2 (labday 146 - 155 )
- October part 3 (labday 156 - 166 )
- November (labday 167 - 170 )
136. labday 01.10.2010
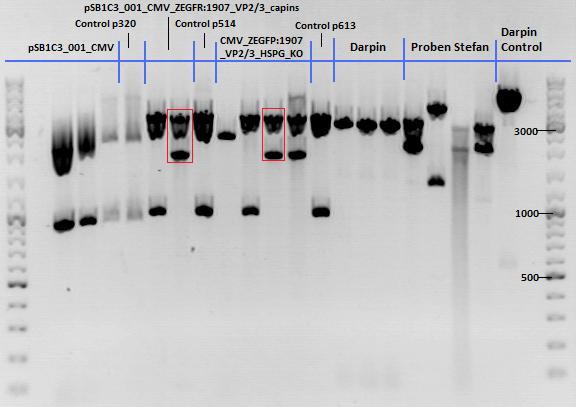
Test Digestions of yesterdays minipreps
Investigator: Achim, Anna
- 001_CMV: Cut with EcoRI, PstI & PvuII
- P654 (Lane 1)
- P655 (Lane 2)
- P686 (Lane 3)
- Control P320 (Lane 4)
- CMV_affi_VP2/3_capins: Cut with EcoRI & SspI
- P678 (Lane 5)
- P679 (Lane 6)
- Control P514 (Lane 7)
- Results:The expected bands should run at 1700 and 3000 bp. P679 was sent for sequencing.
- CMV_affi_VP2/3_capins_HSPG-KO: Cut with EcoRI & SspI
- P680 (Lane 8)
- P681 (Lane 9)
- P684 (Lane 10)
- P685 (Lane 11)
- Control P613 (Lane 12)
- Results:The expected bands should run at 1700 and 3000 bp. P684 was sent for sequencing.
- pCerulean_Vp1up_NLS_Darpin: Cut with BamHI & XbaI
- P676 (Lane 13)
- P677 (Lane 14)
- P683 (Lane 15)
- Control P365 (Lane 20)
- Results:The expected bands should run at 500 and 4500 bp. However, just one band at ~3000 bp is visible on the gel.
Samples Stefan: Cut with Xba & Spe
- P670 (Lane 16)
- P671 (Lane 17)
- P672 (Lane 18)
- P673 (Lane 19)

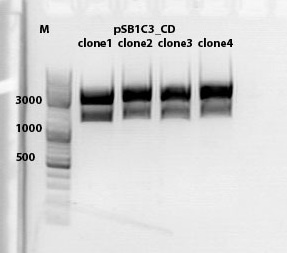
mini preps of CD clones
Investigator: Kira
c(p689)= 115 ng/ul
c(690)= 151, 20 ng/ul
c(691)= 122,27 ng/ul
c(p692) = 126,42 ng/ul
test digestion of CD clones
Investigator: Kira
| Components | sample Volume/µL |
| DNA | 4,0 µl |
| BSA (10x) | 2 µl |
| Buffer no. 4 | 2,0 µl |
| Enzyme 1 XbaI | 1,0 µl |
| Enzyme 2 AgeI | 1,5 µl |
| H2O | 9,5 µl |
| Total volume | 20 |
incubation @ 37 C for approx. 2 h
1% agarose gel
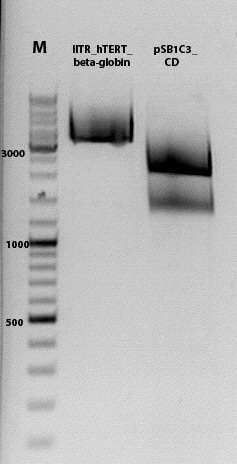
Biobrick assembly pSB1C3_lITR_hTERT_beta-globin_CD
Investigator: Kira
c(pSB1C3_lITR_hTERT_beta-globin)= 333 ng/ul
c(pSB1C3_CD)= 151 ng/ul
| Components | vector Volume/µL | insert Volume/µL |
| DNA | 4,5 µl | 6 |
| BSA (10x) | 3 µl | 3 |
| Buffer no. 4 | 3,0 µl | 3 |
| Enzyme 1 XbaI | 0 µl | 1,5 |
| Enzyme 2 SpeI | 1,5 µl | 0 |
| Enzyme 3 PstI-HF | 1,0 | 1 |
| H2O | 17 | 15,5 |
| Total volume | 25 |
incubation @ 37 C for approx. 2 h
1% agarose gel
Ligation
DNA-mix: 8 ul (vector 4,6ul)+(insert 3,4 ul)
T4 ligase: 1 ul
T4 buffer: 1 ul
Incubation @ RT for 30 min
Transformation was performed according to the standard protocol w BL21 cells.
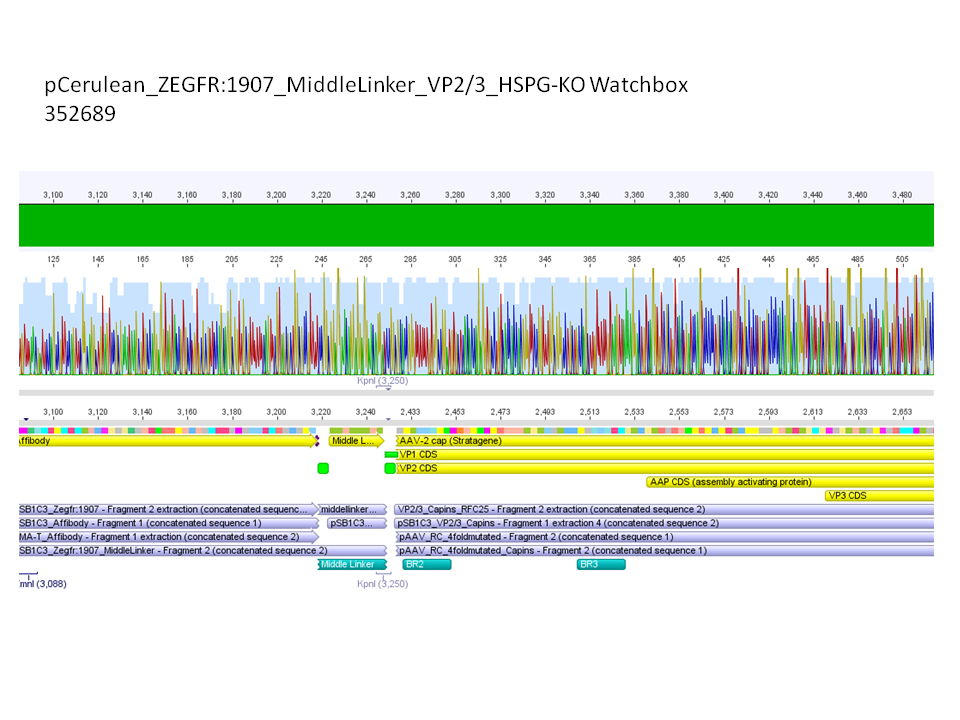
Sequencing results of pCerulean_Zegfr:1907_MiddleLinker_VP2/3_HSPG-KO
Investigator: Hanna
Comment: All N-terminal fusion approaches with VP2/3_HSPG-KO revealed positive results except of pCerulean_Zegfr:1907_MiddleLinker_VP2/3_HSPG-KO. Another clone was picked, preped, test digested and sent for sequencing.
Conclusion: Sequencing results revealed positive results.
Picking clones of pGA14_MiddleLinker_VP2/3_insCap and pGA14_MiddleLinker_VP2/3_HSPG-KO
Investigator: Hanna
Unfortunately there grew a bacteria lawn over night - it was hardly not possible to pick clones. Nevertheless I tried and picked 2 clones of pGA14_MiddleLinker_VP2/3_insCap and pGA14_MiddleLinker_VP2/3_HSPG-KO.
To do: Mini-Prep and test digestion.
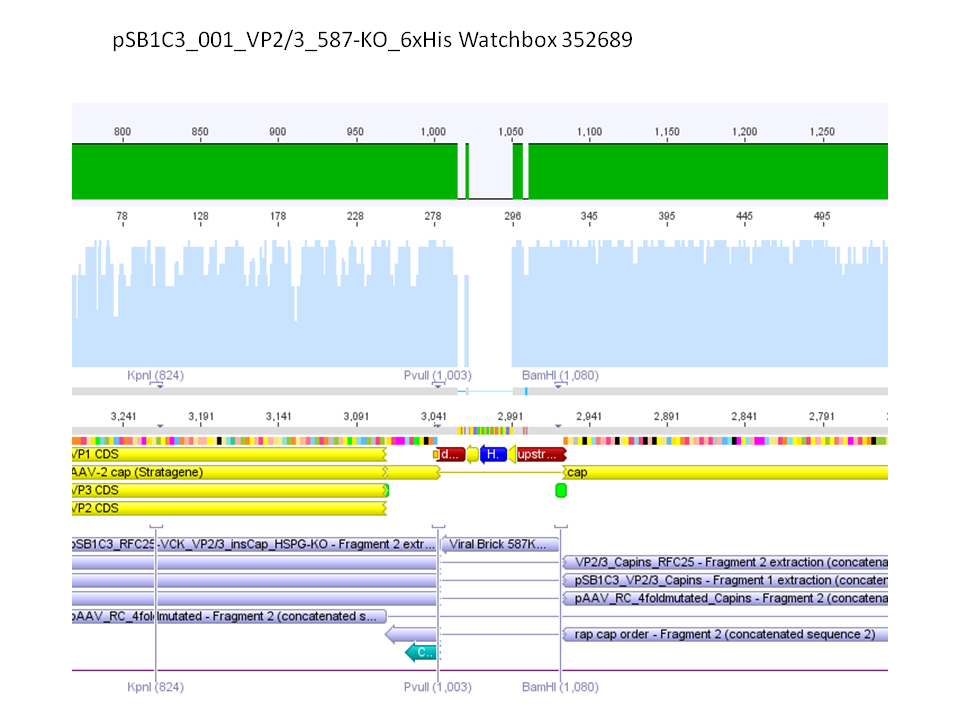
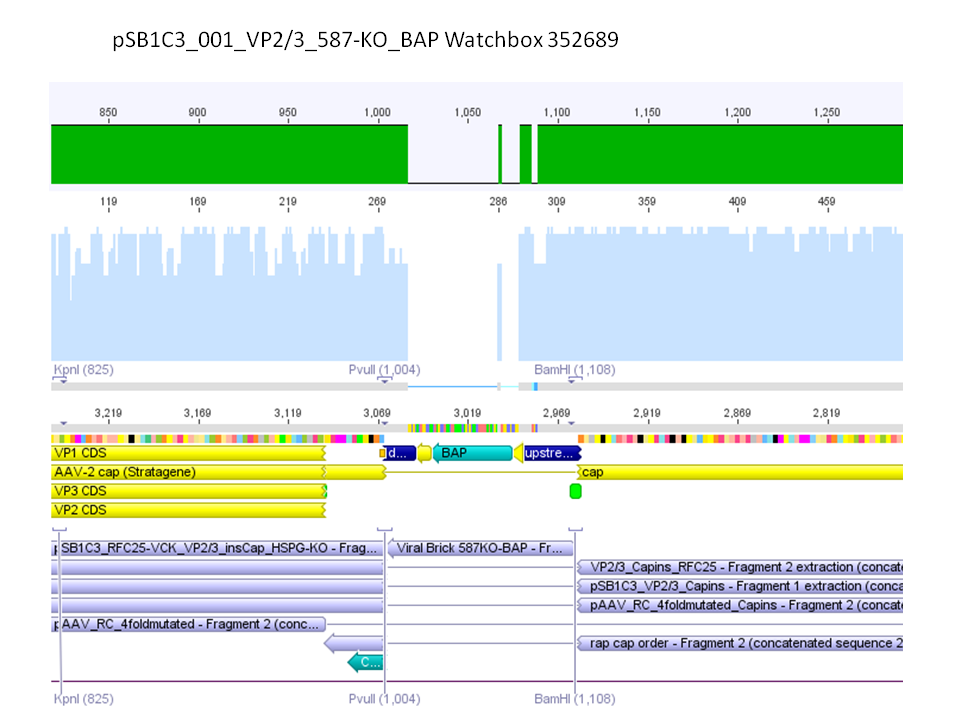
Sequencing results of pSB1C3_001_VP2/3_587-KO_BAP and pSB1C3_001_VP2/3_587-KO_6xHis
Investigator: Hanna
Comment: For the creation of our super constructs, the His-Tag and the BAP motif need to be cloned into VP2/3 for N-terminal fusion to VP2. Sequencing results showed that the 6xHis and BAP motif was not cloned into VP2/3_insCap.
To do: Clone 587-KO_6xHis and 587-KO_BAP into pSB1C3_001_VP2/3_insCap 1. via digestion of inserts and 2. via hybridization of referring oligos - digestion of vector with 800-900 ng.
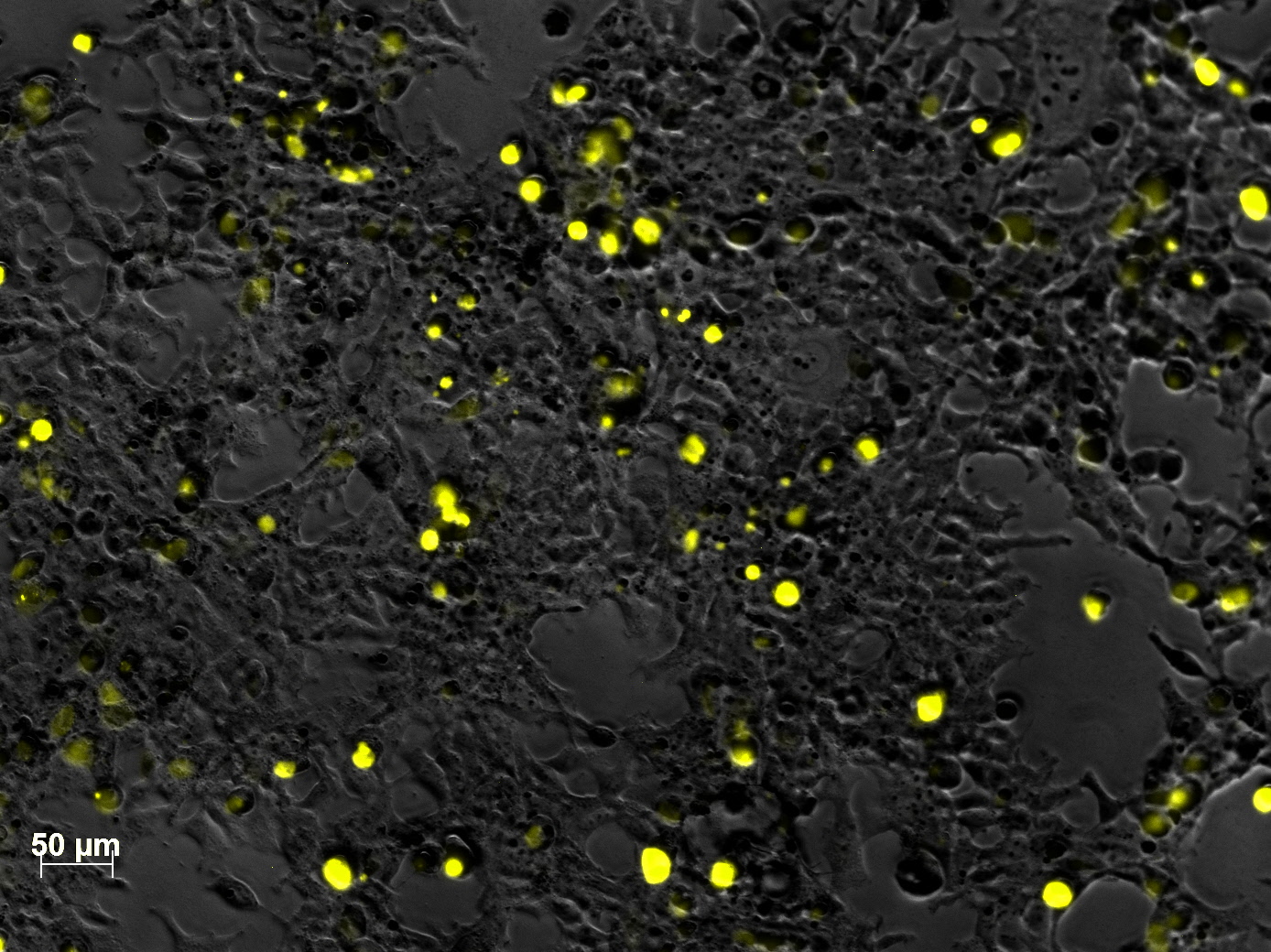
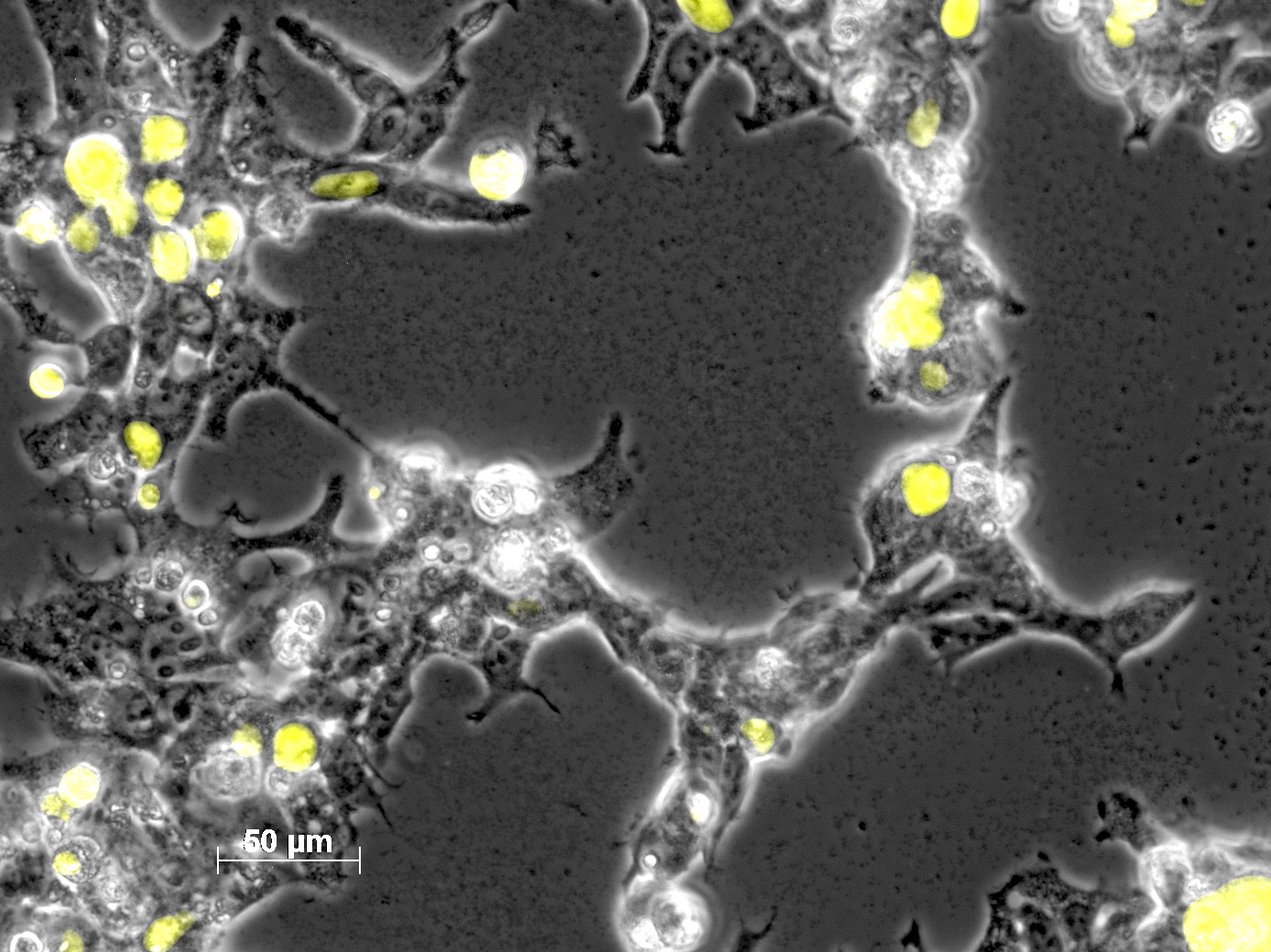
Impressions of transfection of AAV293 with pCerulean_VP1up_NLS_mVenus_VP2/3
Investigator: Adrian
Yesterday AAV293 cells were transfected with pCerulean_VP1up_NLS_mVenus_VP2/3 and GMK-TK30 as gene of interest. Today, nuclear localization of the produced mVenus_VP2/3 fusion protein was visible and can be nicely seen in the following pictures:
Conclusion: The VP2/3 fusion particle was transcribed and translated AND was transported back into the nucleus in order to be packaged by the ITR-flanked gene of interest.
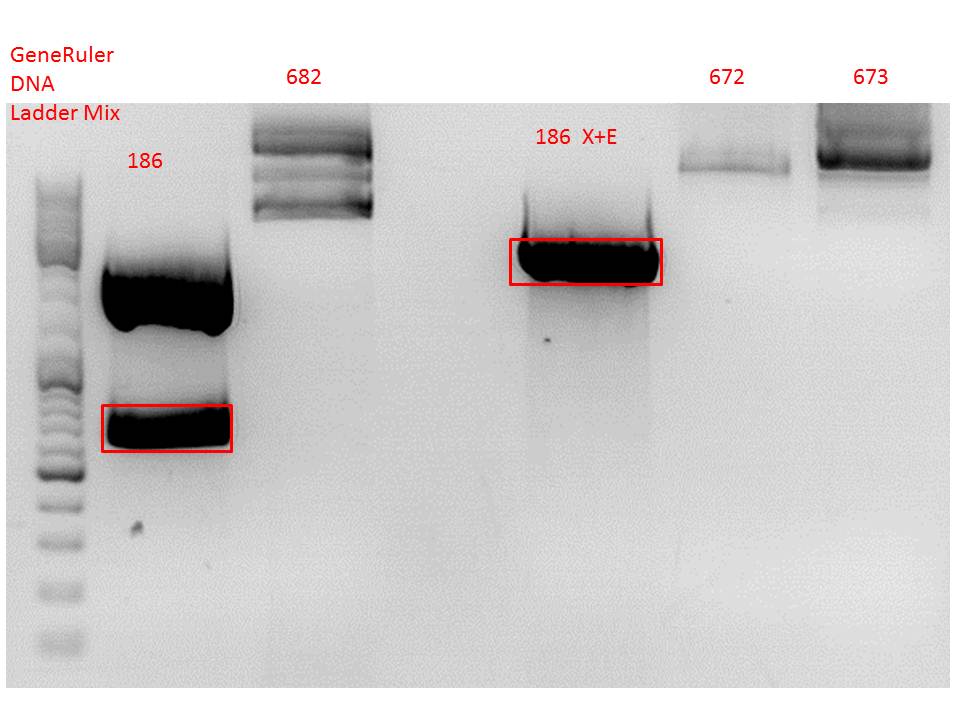
Cloning of hGH_rITR into pSB1C3_lITR_phTERT_beta-globin_CFP and lITR_phTERT_beta-globin_mGMK_TK30 into pSB1C3_hGH_rITR
Investigator: Stefan
Vector name:
- pSB1C3_lITR_phTERT_beta-globin_CFP (P682)
- pSB1C3_hGH_rITR (P186)
Insert name:
- pSB1C3_hGH_rITR (P186)
- pSB1C3_lITR_phTERT_beta-globin_mGMK_TK30 (P672)
- pSB1C3_lITR_phTERT_beta-globin_mGMK_TK30 (P673)
| components | volume for P186 /µl | volume of P186 X+E /µl | volume of P672 + P673 /µl | volume of P682 /µl |
| DNA | 12 | 12 | 5 | 14 |
| BSA (10x) | 2,5 | 2 | 2 | 2 |
| Buffer 4 (10x) | 3 | 2 | 2 | 2 |
| Enzyme XbaI | 1 | 1 | - | - |
| Enzyme PstI | 1 | - | - | 1 |
| Enzyme EcoI | - | 1 | 1 | - |
| Enzyme SpeI | - | - | 1 | 1 |
| H2O | 6 | 6 | 9 | - |
| Total volume (e.g. 15,20,25,30 µl) | 25 | 25 | 20 | 20 |
Gel:
0,5 g Agarose, 50 ml TAE (1%), 3 µl GELRED , at 115 Volt
Comment: Since the first gel revealed problems with samples 672, 673 and 682 another digestion was prepared (same approach as shown above), this time using a 0,8% gel and another loading dye (without SDS).
0,4 g Agarose, 50 ml TAE (0,8%), 3 µl GELRED , at 115 Volt
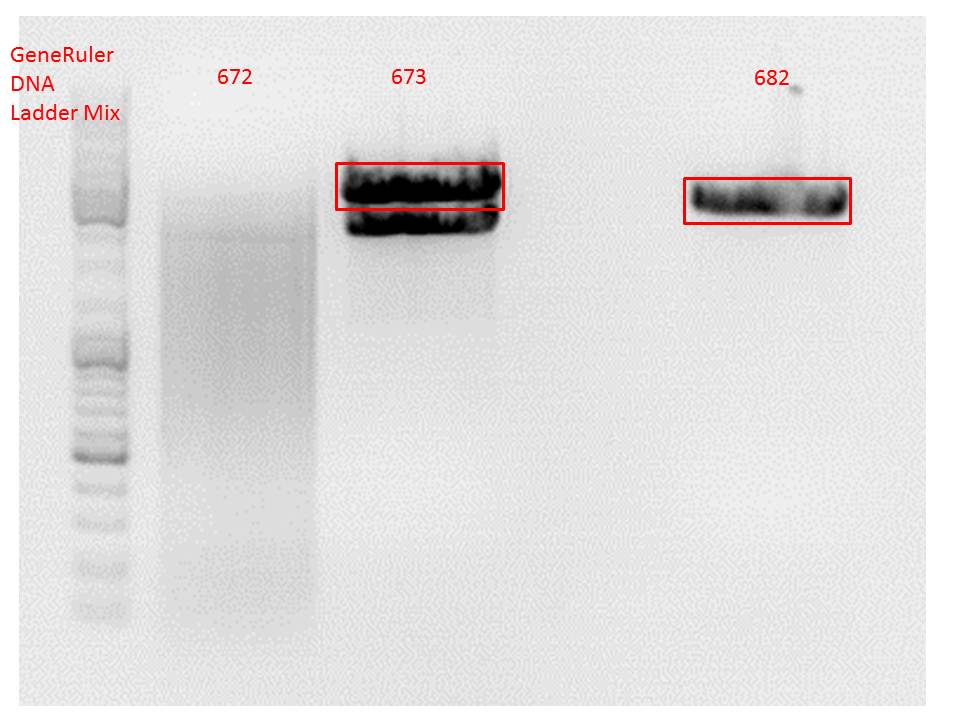
Comment: Since sample 672 showed no bands this sample was discarded and cloning continued only using 673.
Gel extraction:
Was performed according to protocol.
T4 Ligation:
Ligation was performed over night.
| ligation name | 186 (X+E) + 673 | 186 + 682 |
| volume of vector | 3,51 | 4,49 |
| volume of insert | 5,38 | 2,64 |
Transformation:
Will be done tomorrow using BL21 cells.
New BL21 cells ready to use!
Investigator: Stefan
137. labday 02.10.2010
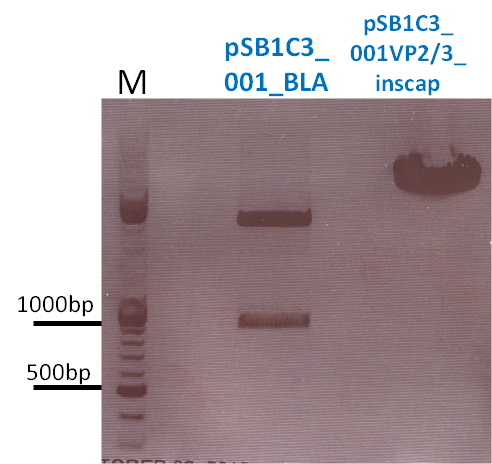
Repetition of the approach of subcloning the ViralBricks HSPG-ko BAP and His into VP2/3 with different strategies
Investigator: Bea
Comment: Unfortunately, the last experiment revealed that subcloning of the two ViralBricks did not work out which was shown by the sequencing results. Therefore we need to repeat the approach of subcloning the ViralBricks into the pSB1C3_VP2/3 which than can be used for fusing it to the n-terminal targeting approaches. Two different strategies will be carried out which means that we are digesting the pSB1C3_Viralbricks AND hybridize the oligos (BAP and His) in order to obtain some positive results.
The vector and ViralBricks were digested with BamHi and PvuII. The vector was dephosphorylated following the standard protocol.
The vector was loaded on a 1% agarose gel before dephosphorylation was conducted. The results can be seen above in the gel picture:
Result:
The ligation was performed over night at 18°C with T4-ligase.
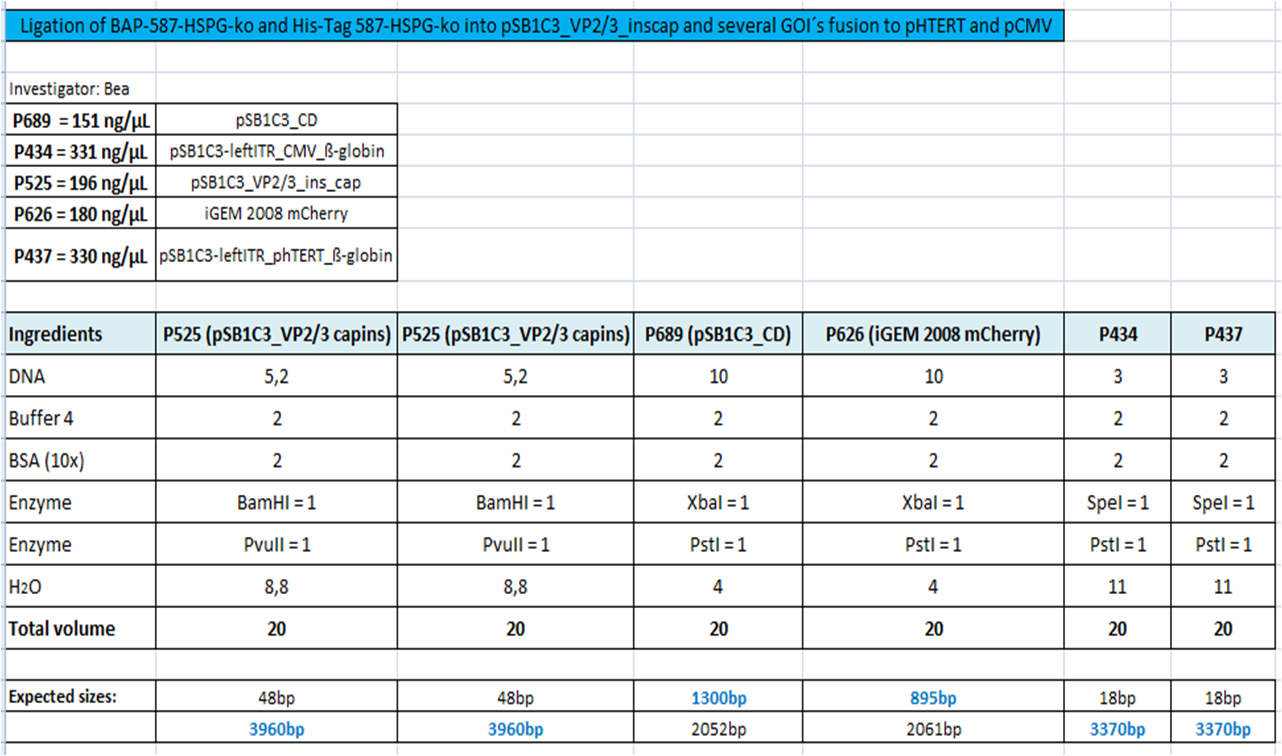
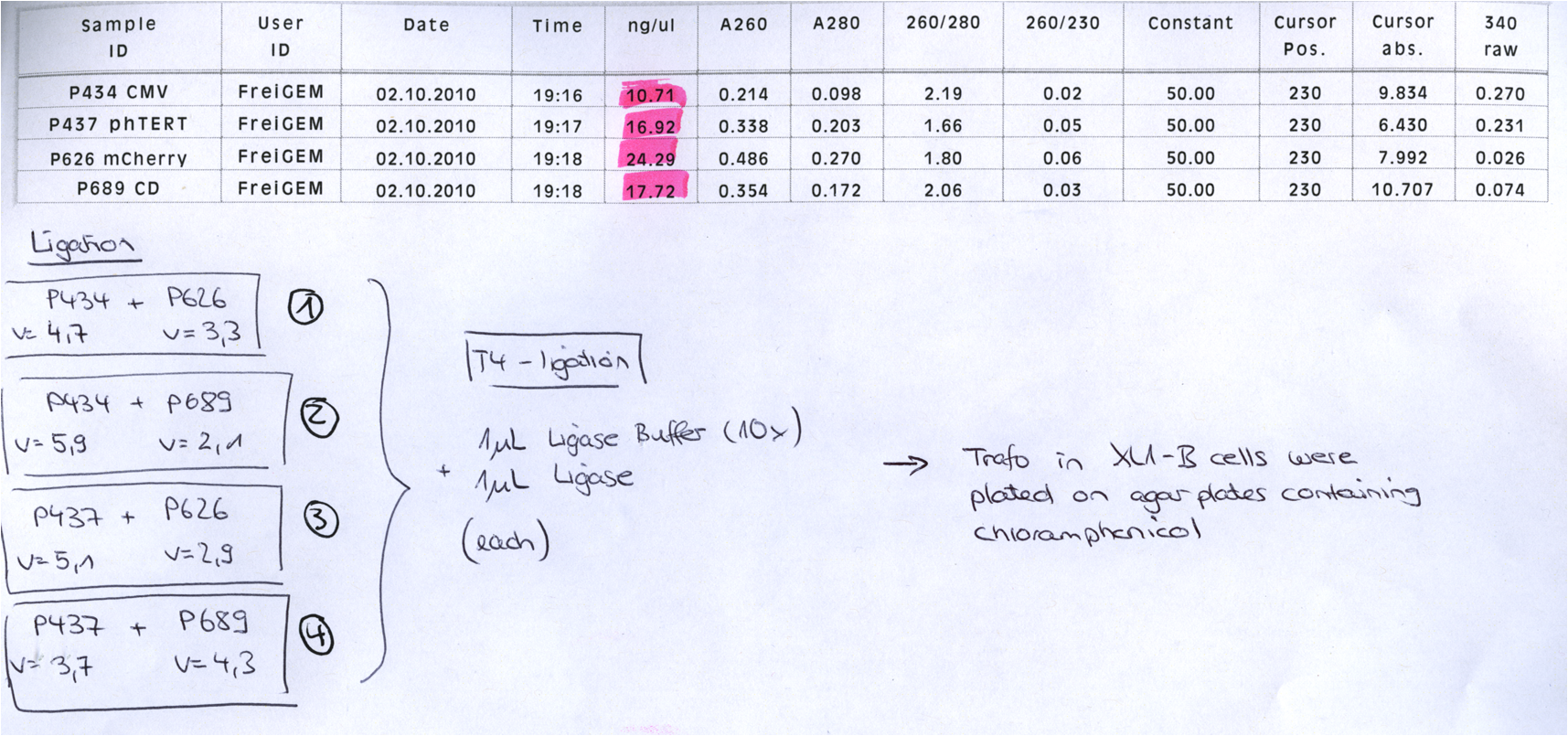
BioBrick Assembly of CD and mCherry
Investigator: Bea
Comment: We decided to choose some additional GOI´s for the vectorplasmid so the "new" GOI´s need to be fused to the leftITR_Promoter_betaglobin constructs. Since only one clone grew on the plate prepared by Kira where she fused the CD (cytosine deaminase) to the above mentioned construct I am going to repeat it aswell.
Digestion of the constructs:
- P689 = pSB1C3_CD
- P626 = iGEM_mCherry
- P434 = pSB1C3_leftITR_CMV_betaglobin
- P434 = pSB1C3_leftITR_phTERT_betaglobin
- P525 = pSB1C3_VP2/capins
Loading plan:
P689 (pSB1C3_CD) P626 (iGEM mCherry) P434 (pSB1C3_lITR_CMV_ß-globin) P437 (pSB1C3_lITR_phTERT_ß-globin)
Results:
After gel extraction has been performed, the ligation was carried out.
Ligation:
The ligation mix was transformed into XL1-Blue cells and plated on agar plates containing chloramphenicol.
Next steps:
Picking clones and perform Mini-Prep. After the Mini-Preps have been performed this construct needs to be sequenced and tested in cell culture.
comment: just one clone? uhhhh, when did you take out the plate? i put it at around 1 am..maybe they need a while to grow, no? (Kira)
Yep, I found another clone ;-) But the two look pretty good. I took them out today in the afternoon - I guess that is enough time! But if you want I can put them back into the 37° C room??! (Bea)
Mini Prep and test digestion of pGa14_MiddleLinker_VP2/3_HSPG-KO
Investigator: Hanna
Comment: In order to fuse the DARPin to the N-terminus of VP2/3_insCap and VP2/3_HSPG-KO, these motifs need to be fused to the Middle Linker (performed on 30.9.2010). In order to be able to immediately continue with cloning, BL21 cells were transformed and clones were picked in the noon. Now, the DNA needs to be preped and test digested in order to perform a 3-fragment-ligation with the DARPin and the pSB1C3_001_CMV plasmid. Unfortunately the cells of the VP2/3_insCap construct grew not densely enough. Therefore two new clones were picked from the plate and were inoculated in 10 mL LB medium. The other construct (pGa14_MiddleLinker_VP2/3_HSPG-KO) was preped and test digested.
TEST DIGESTION:
For both clones:
- DNA: 4 µL
- Buffer 4: 1 µL
- BSA: 1 µL
- EcoRI: 0.5 µL
- PstI: 0.5 µL
- H2O: 3 µL
Incubation: 1 hour.
Agarose gel: 0.8 %, 115 Volt, 1 hour.
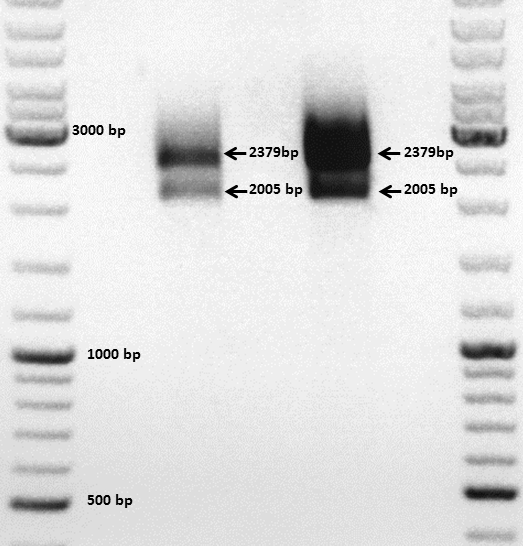
Comment: The choice of these restriction enzymes was not clever, because if cloning didn't work, I expect one band at 2379 bp (the insert should hardly be seen) - if it worked one band at 2379 bp and a second at 2005 bp. Fortunately the bands could be separated ans revealed positive results for both clones :
Next steps:
- Mini-Prep and test digestion of pGa14_MiddleLinker_VP2/3_ins Cap.
- 3 fragment ligation of MiddleLinker_VP2/3_ins Cap or MiddleLinker_VP2/3_HSPG-KO + DARPin + pSB1C3_001_CMV backbone.
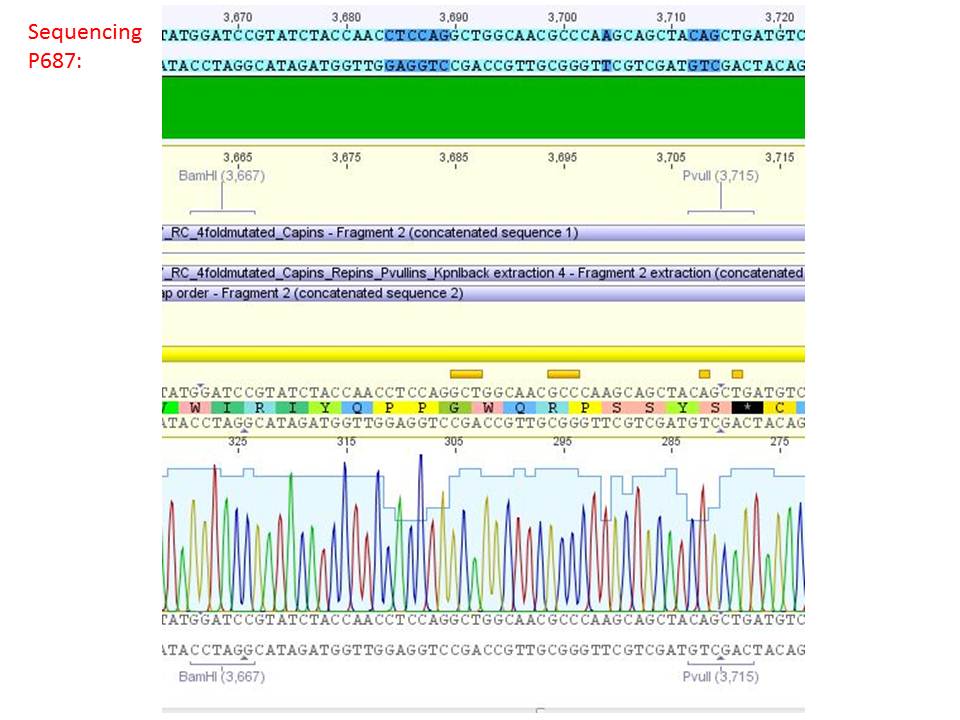
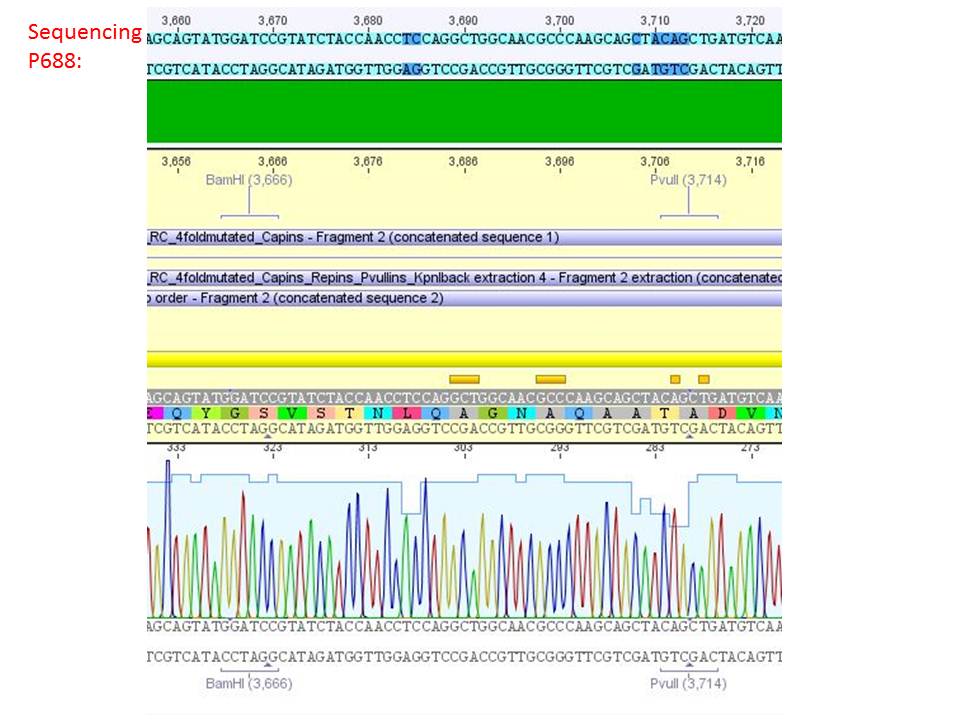
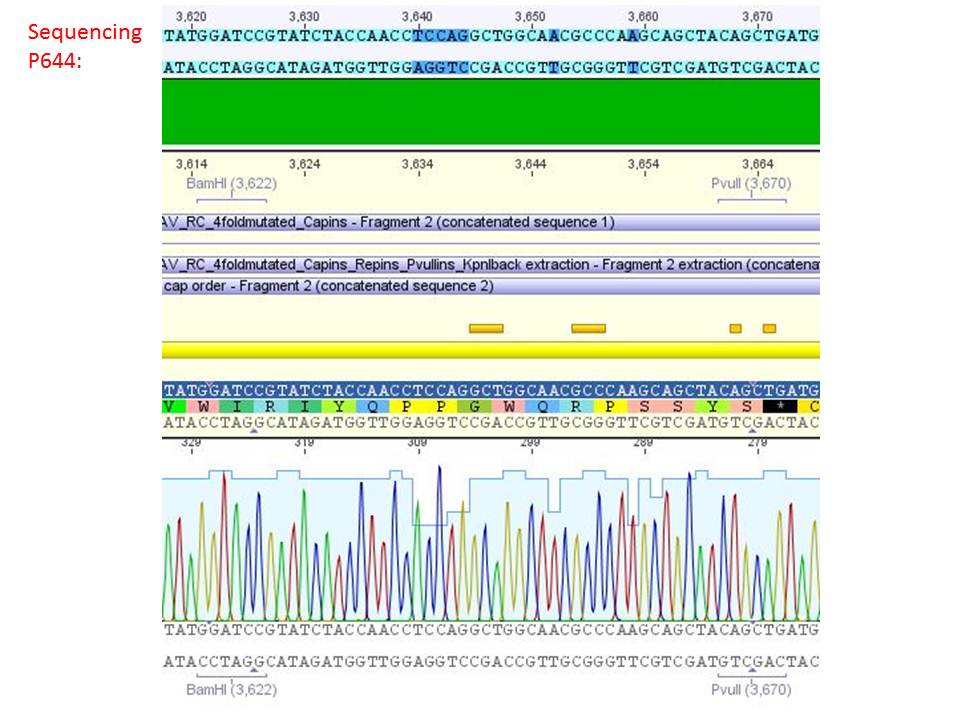
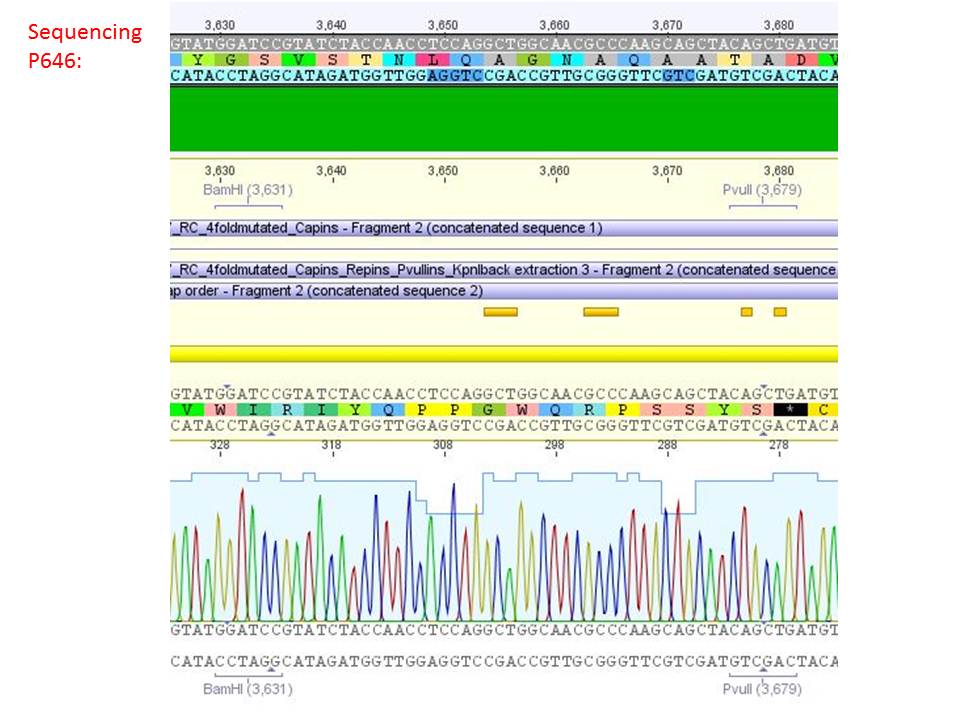
Sequencing analysis of HSPG-ko in several IRCK constructs
Investigator: Stefan
pSB1C3_001_RC_IRCK_HSPG-ko_P5tataless_RFC10 clone 1 (P687):
pSB1C3_001_RC_IRCK_HSPG-ko_P5tataless_RFC10 clone 2(P688):
pSB1C3_001_RC_IRCK_VP1-ko_HSPG-ko_P5tataless cl1 (P644):
pSB1C3_001_RC_IRCK_VP2-ko_HSPG-ko_P5tataless cl1 (P646):
Comment: Sequencing results show that all plasmids contain the HSGP-ko. Working will be continued using P644, P646 and P687.
Mini Prep of pGa14_MiddleLinker_VP2/3_inscap and pSB1C3_Darpin
Investigator: Jessica
- pSB1C3_Darpin clone1 c=158.5 ng/µl
- pSB1C3_Darpin clone2 c=205.6 ng/µl
- pGA14_MiddleLinker_VP2/3_inscap clone 1 c=147.5 ng/µl
- pGA14_MiddleLinker_VP2/3_inscap clone 2 c=121.0 ng/µl
Test-digestion:
| Components | pSB1C3_Darpin clone1/2 |
| DNA | 1,5 µl |
| BSA (10x) | 1 µl |
| Buffer no. 4 | 1 µl |
| Enzyme 1 XbaI | 0,4 µl |
| Enzyme 2 AgeI | 0,4 µl |
| H2O | 5,7 µl |
| Total volume | 10 |
Hanna wrote that a 3rd enzyme is need for the test digestion of pGA14_MiddleLinker_VP2/3_inscap but I couln't find any sequence of pGA14_MiddleLinker_VP2/3_inscap or pGA14

Test digestion of pGA14_MiddleLinker_VP2/3_inscap
Investigator: Achim
- pGA14_MiddleLinker_VP2/3_inscap clone 1 c=147.5 ng/µl (P698)
- pGA14_MiddleLinker_VP2/3_inscap clone 2 c=121.0 ng/µl (P699)
- Control: pGA14_MiddleLinker (P301)
- Digestion with EcoRI & SalI; should yield two bands of 3400 & 1000 bp. The negative control should only be cut once.
Mass Midi-Prep III
Investigator: Chris W.
Midi-Prep of:
pSB1C3_001_RC_IRCK_VP1-ko_HSPG-ko_P5tataless cl1 =P700 =B521
pSB1C3_001_RC_IRCK_VP2-ko_HSPG-ko_P5tataless cl1 =P701 =B523
pSB1C3_001_RC_IRCK_HSPG-ko_P5tataless_RFC10 clone 1 =P702 =B561
pCerulean_ZEGFR:1907_MiddleLinker_VP2/3_HSPG-KO clone 2 =P703 =B543
pCerulean_CFP_MiddleLinker_VP2/3_HSPG-KO =P704 =B507
pCerulean_ZEGFR:1907_SEG_VP2/3_HSPG-KO =P705 =B509
pCerulean_ZEGFR:1907_ShortLinker_VP2/3_HSPG-KO =P706 =B510
pCerulean_ZEGFR:1907_LongLinker_VP2/3_HSPG-KO =P707 =B511
pCerulean_6xHis_MiddleLinker_VP2/3_HSPG-KO =P708 =B512
pCerulean_VP1up_NLS_ZEGFR:1907_VP2/3_HSPG-KO =P709 =B513
pCerulean_VP1up_NLS_6xHis_VP2/3_HSPG-KO =P710 =B514
pCerulean_VP1up_NLS_mVenus_VP2/3_HSPG-KO =P711 =B515
pSB1C3_001_RC_IRCK_P5tataless clone 1 =P712 =B516
The Midi-Preps were performed according to the standard protocol yielding the following concentrations:
| plasmid-no. | P700 | P701 | P702 | P703 | P704 | P705 | P706 | P707 | P708 | P709 | P710 | P711 | P712 |
| concentration (ng/µl) | 695 | 370 | 601 | 1548 | 3996 | 3630 | 2460 | 2610 | 1860 | 2913 | 2964 | 3030 | 1200 |
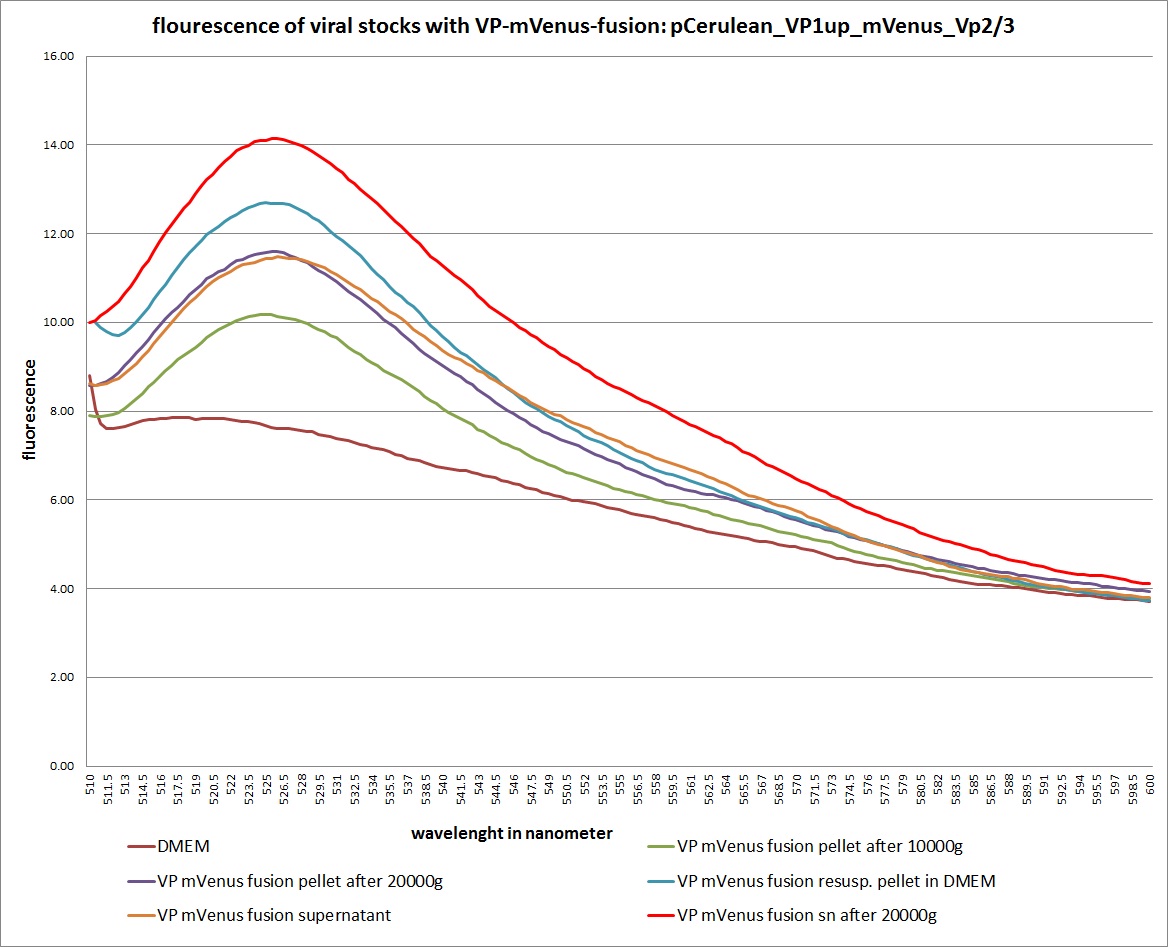
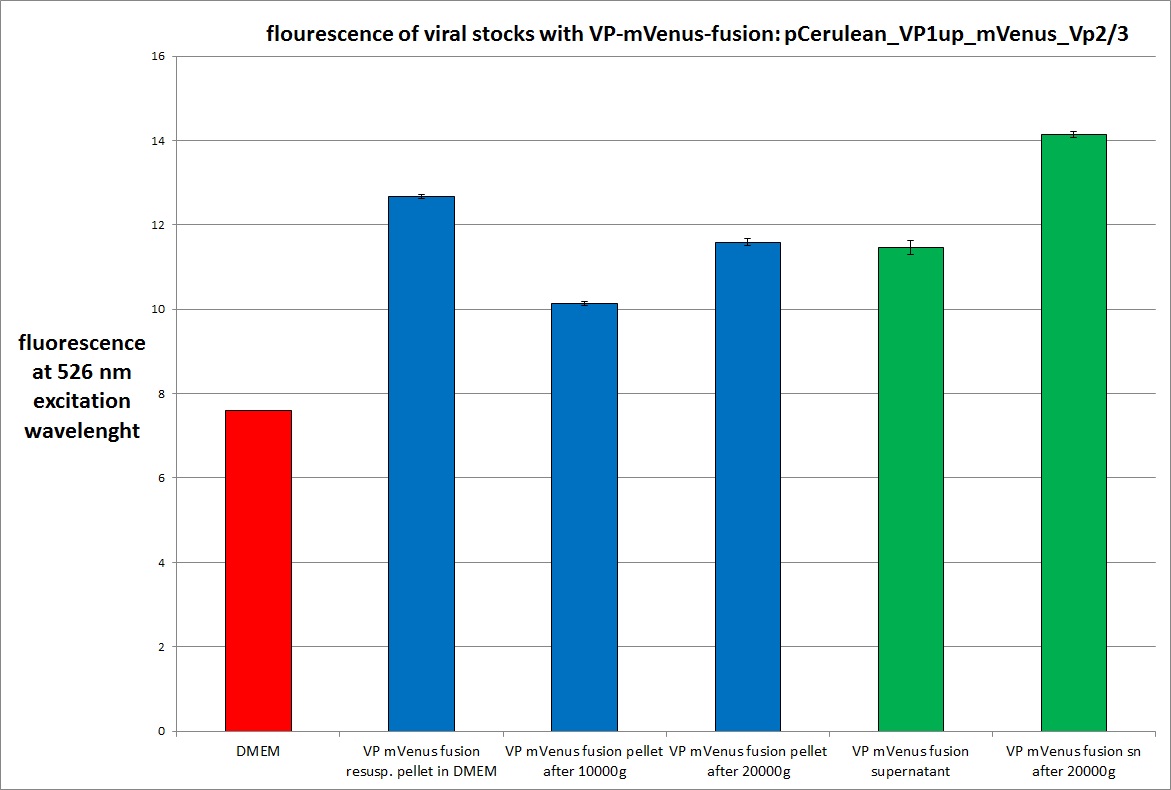
Analysis of viral harvest via spectrometer
Investigator: Adrian, Patrick
The motivation: We want to figure out if it is possible to purify our viral stocks via centrifugation with 10.000 g and/or 20.000 g. In theory/ according to the literature the viral particles should be in the cells and attached to the HSPGs at the cellular surfaces.
The plan: Two different viral stocks were prepared:
Stock 1 with VP2-mVenus-fusion-Capsid loaded with TKGMK (4 wells from a 6 well plate)
- R/C 1: 50% pCerulean_VP1up_NLS_mVenus_Vp2/3 P501
- R/C 2: 50% Rep/Cap2: P449 pAAV_RC_4fachmut_VP1-ko:
- GOI: TKGMK: P82.b
- pHelper
Stock 2 "standard" virus (4 wells from a 6 well plate)
- R/C 1 P486
- GOI: TKGMK: P82.b
- pHelper
These two stocks got harvested according to standard protocol (scratching cells, transfer them in to 15 ml falcons, resuling 12ml solution total). After centrifugation (10 min 200g), the supernatant got transferred into two new 15 ml falcons and the pellets were resuspended with 10 ml DMEM Medium. The Stocks got freezed thawed two times so finally there were:
- DMEM as negative control
- resuspended Pellet with mVenus-fusioned viral capsids
- resuspended Pellet with "standard virus"
- supernatant with mVenus-fusioned viral capsids
- supernatant with "standard virus"
The fluorescence of each stock was measured via spectrometer. After that, stocks 2 and 4 were centrifugated 10min with 10.000 g followed by an other fluorescence measuring. Finally the stocks got spin down with 20.000 g for 10 min
The results:
The conclusions:
The highest amount of fluorescence was measured in the supernatant, the individual centrifugation steps had no decrease in fluorescence as consequence!
Keep in mind, without further investigation it is not valid to say that we can purify our stocks via 10-20.000 g centrifugation steps, because we dont know if the viral capsids are still intact! The FACS analysis are not sufficient enough to make a decision, because our last samples had YFP as GOI (=> so efficient cell sorting was not possible). We need to do qPCRs to make a valid evidence.
200µl-Transduction for testing the pTERT-promoter
Investigator: Adrian, Patrick
We transduced A431, HT1080 and AAV293 cells with 4 different viral stocks:
- control vector from 15.9, 750.000 cells, 3.3 µg approach
- P25 R/C= 326 pTERT_mVenus
- P26 pAAV_RC_RepCapIns_SDMKpnI clone 1 P431 pTERT_mVenus
- His-linker-fusion
CRUCIAL: There was not enough viral stock: so the whole Transduction was performed with 200µl instead of 1000µl
There are 45 samples to FACS on monday 4.10. Tripple values for each stock of each cell lines.
Transfection
Investigators: Adrian, Patrick
Transfection followed standard protocol (3.3 µg of each plasmid, 1 ml 0.3M CaCl2 and 1 ml of 2xHBS buffer PH 11.12) but with 20 min incubation time (Ca-DNA-2xHBS)
following stocks:
- mVenus standard stock 10 x cm2 plates
- TKGMK stock 10 x cm2 plates
- viral particles with GOI (mVenus) missing HGH one 6well plate
- viral particles with GOI (mVenus) missing beta globin one 6well plate
- viral particles with R/C missing HSPG binding motif one 6well plate
- viral particles with new standard R/C one 6well plate
138. labday 03.10.2010
Proceeding with the approach of subcloning the ViralBricks HSPG-ko BAP and His into VP2/3 with different strategies
Investigator: Bea
Comment: Ligation was carried out over night and transformation will be performed today.
Since the digested vector was not sufficient for all four ligation I decided to perform the ligation only with the hybridized oligos 587-ko His and the digested Viralbricks 587-HSPG-ko His and BAP. If the digested BAP insert reverals no positive results, the hybrizied oligos for BAP can be used for another approach. The transformation was carried out in
- BL-21 cells
- and plated on agar plastes containing chloramphenicol
Midi-Prep of pSB1C3_lITR_CMV_betaglobin_mVenus_hGH_rITR clone1
Investigator: Chris W.
Midi-Preps of pSB1C3_lITR_CMV_betaglobin_mVenus_hGH_rITR clone1 =P713 =B200
The Midi-Prep were performed according to the standard protocol yielding the following concentrations:
| plasmid-no. | P713 |
| concentration (ng/µl) | 1248,3 |
139. labday 04.10.2010
´Seeding AAV293 for Transfection
Investigator Patrick
9 6-well plates were prepared for the transfection tomorrow. 480000 cells were seeded into each well.
FACS Analysis of pTERT_mVenus-loaded-viral particles and pCerulean_VP1up_His_VP2/3
Investigators: Kerstin, Anissa
45 samples of the three cell lines: AAV293, A431 and HT1080 got transduced (see 2.10) with following viral stocks:
1.
- pHelper
- R/C : 326
- pTERT-mVenus
2.
- pHelper
- R/C : P431
- pTERT-mVenus
3.
- pHelper
- R/C P158a
- CMV_mVenus
4.
- pHelper
- R/C: pCerulean_VP1up_His_VP2/3
- pAAV_RC_4fachmut_VP1-ko
- CMV_mVenus
Assembly of pSB1C3_CMV_DARPin_VP2/3_insCap and pSB1C3_CMV_DARPin_VP2/3_HSPG-KO
Investigators: Hanna
Comment: We also want to test the DARPin_E01 for the N-terminal fusion approaches. Today a 3 fragment ligation will be performed in order to complete the VP2 fusion constructs with the DARPin.
Because 2 test digestions showed that the fusion of the DARPin to VP1up_NLS didn't work as ecpected (insert is too large) this has to be repeated before also the VP1 insertion with the DARPin can be completed.
Digestion
| components | volume of pSB1C3_CMV /µl | volume of pG14_ML_VP2/3_insCap /µl | volume of pG14_ML_VP2/3_HSPG-KO /µl | volume of pSB1C3_001_DARPin /µl |
| DNA | 9 | 6.8 | 10 | 5 |
| BSA (10x) | 2 | 3 | 3 | 2 |
| Buffer 4 (10x) | 2 | 3 | 3 | 2 |
| SpeI | 1 | - | - | - |
| PstI | 1 | 1 | 1 | - |
| NgoMIV | - | 1 | 1 | - |
| XmnI | - | 1 | 1 | - |
| XbaI | - | - | - | 4 |
| H2O | 5 | 14.2 | 11 | 9 |
| Total volume | 20 | 30 | 30 | 20 |
- Incubation: 2.5 h
Agarose-Gel:
0.5 g Agarose, 50 mL TAE (1 %), 3 µL GELRED, at 115 Volt, running time: 45 minutes
 "
"