Team:Stockholm/28 August 2010
From 2010.igem.org
(Difference between revisions)
(→Andreas) |
(→Andreas) |
||
| Line 36: | Line 36: | ||
Elution volume: 30 μl (eluted twice to increase DNA yield) | Elution volume: 30 μl (eluted twice to increase DNA yield) | ||
*Pur. Dig. pMA His E+N | *Pur. Dig. pMA His E+N | ||
| + | |||
| + | ===Gel verification=== | ||
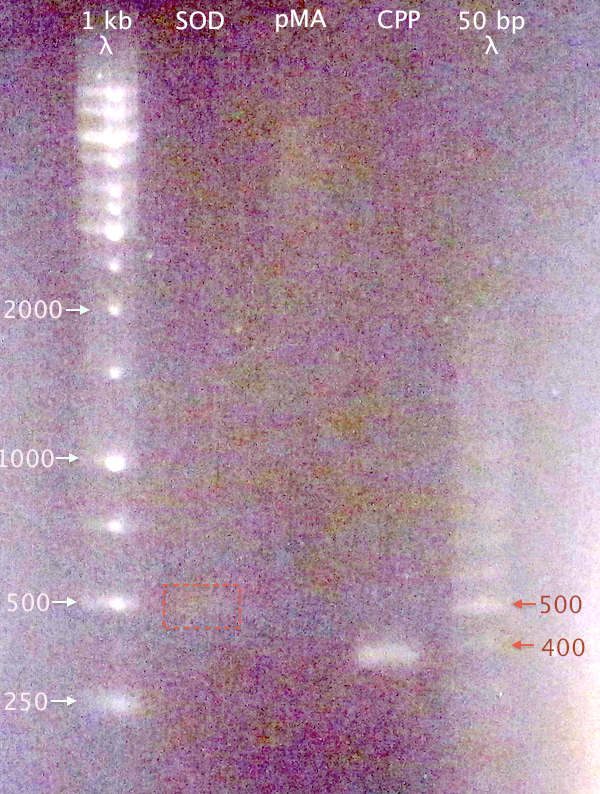
| + | [[image:Gelver_DNAextr_28aug.png|200px|right|thumb|'''Gel verification of gel extracted and purified DNA samples.'''<br />3 μl λ; 2 μl sample.<br />1 kb λ=O'GeneRuler 1 kb DNA ladder. 50 bp λ=GeneRuler 50 bp DNA ladder.]] | ||
| + | Since no DNA content was measurable for "Extr. Dig SOD E+A", a gel was run to verify DNA content in the three purified samples. | ||
| + | |||
| + | 1 % agarose, 110 V | ||
| + | |||
| + | Samples: | ||
| + | *SOD: Extr. Dig SOD E+A | ||
| + | *pMA: Pur. Dig pMA.His E+N | ||
| + | *CPP: Extr. N-CPP cluster | ||
| + | |||
| + | Expected bands: | ||
| + | *SOD: 492 bp | ||
| + | *pMA: 2427 bp | ||
| + | *CPP: 379 bp | ||
| + | |||
| + | ====Results==== | ||
| + | *CPP resulted in a clear band of correct size. | ||
| + | *A very weak band at ≈500 bp is visible for SOD, which might be traces of DNA. | ||
| + | *No band visible for pMA. | ||
| + | |||
| + | Nevertheless, proceeded to ligation and cloning, hoping that the DNA is there. | ||
| + | |||
| + | ===Cloning of SOD into pMA.His=== | ||
| + | |||
| + | ====Ligation==== | ||
| + | {|border="1" cellpadding="1" cellspacing="0" | ||
| + | | | ||
| + | ![μl] | ||
| + | |- | ||
| + | |Extr. Dig SOD E+A | ||
| + | |align="center"|12 | ||
| + | |- | ||
| + | |Pur. Dig pMA.His E+N | ||
| + | |align="center"|3 | ||
| + | |- | ||
| + | |5X Rapid Ligation buffer | ||
| + | |align="center"|4 | ||
| + | |- | ||
| + | |T4 DNA ligase | ||
| + | |align="center"|1 | ||
| + | |} | ||
| + | |||
| + | Incubation: 22 °C, 10 min | ||
| + | |||
| + | ====Transformation==== | ||
| + | *3 μl ligation mix. 30 min on ice. | ||
| + | *30 sec heat shock in 42 °C | ||
| + | *Cells grown on Amp 100 LB agar, 37 °C | ||
| + | |||
| + | ===Amplification of N-CPPs from N-CPP cluster=== | ||
Revision as of 12:09, 30 August 2010
Contents |
Andreas
Gel extraction
| DNA concentrations | ||
|---|---|---|
| Sample | Conc [ng/μl] | A260/A280 |
| Extr. N-CPP | 40.91 | 1.69 |
| Extr. Dig SOD E+A | – | – |
| Pur. Dig pMA.His E+N | 36.02 | 1.80 |
From 27/8
Purified DNA from excised samples "Extr. Dig. SOD E+A" and "Extr. N-CPP cluster" using the E.Z.N.A. Gel extraction kit; procedures according to provided protocol.
Elution volume: 30 μl (eluted twice to increase DNA yield)
- Extr. N-CPP
- Extr. Dig SOD E+A
DNA purification of "Dig. pMA.His (E+N)"
From 27/8
DNA clean-up using the E.Z.N.A. Gel Extraction kit, following procedures for DNA purification.
Elution volume: 30 μl (eluted twice to increase DNA yield)
- Pur. Dig. pMA His E+N
Gel verification
Since no DNA content was measurable for "Extr. Dig SOD E+A", a gel was run to verify DNA content in the three purified samples.
1 % agarose, 110 V
Samples:
- SOD: Extr. Dig SOD E+A
- pMA: Pur. Dig pMA.His E+N
- CPP: Extr. N-CPP cluster
Expected bands:
- SOD: 492 bp
- pMA: 2427 bp
- CPP: 379 bp
Results
- CPP resulted in a clear band of correct size.
- A very weak band at ≈500 bp is visible for SOD, which might be traces of DNA.
- No band visible for pMA.
Nevertheless, proceeded to ligation and cloning, hoping that the DNA is there.
Cloning of SOD into pMA.His
Ligation
| [μl] | |
|---|---|
| Extr. Dig SOD E+A | 12 |
| Pur. Dig pMA.His E+N | 3 |
| 5X Rapid Ligation buffer | 4 |
| T4 DNA ligase | 1 |
Incubation: 22 °C, 10 min
Transformation
- 3 μl ligation mix. 30 min on ice.
- 30 sec heat shock in 42 °C
- Cells grown on Amp 100 LB agar, 37 °C
 "
"