Team:LMU-Munich/Notebook/Apoptosis
From 2010.igem.org
(→8-14-2010) |
(→8-14-2010) |
||
| Line 231: | Line 231: | ||
== 8-14-2010 == | == 8-14-2010 == | ||
- transfer PhiC31o culture to new LB medium + Amp, 37°C | - transfer PhiC31o culture to new LB medium + Amp, 37°C | ||
| - | - pick up | + | |
| + | - pick up CMV and pDS7 colonies from plates and transfer to LB medium+Amp, 37°C | ||
== 8-15-2010 == | == 8-15-2010 == | ||
Revision as of 09:59, 16 August 2010
Some test text in bold
We created following tests:
- test4 Example of a table
this too is a table:
table with 3 cells
text
text
test text Knallroter Text test grüner text
Transforming competent cells
- eGFP Biobrick: BBa_I714891 SDY_eGFP (Kanamycin)
- TEV recogn N Degron SF3 = pDS7 (Ampicillin)
- TEV p14 recogn = 190-6 (Ampicillin)
-> Protocol: (3 Transformation)
- We added 2 µl DNA
- We plated out 200 µl
- CMV-Promoter Biobrick: BBa_J52034
-> Protocol:(4 Plasmid extraction from cells)
- Prepared overnight culture, measured concentration of DNA
-> Poor results -> thrown away
New Plasmid Extraction
- CMV-Promoter Biobrick: BBa_J52034
-> Protocol: (4 Plasmid extraction from cells)
- Plasmid concentration: 143ng/µl
- 3 ml LB-Media + 4 µl Kanamycin
- Inoculated iangeimpft) with 1 colony of BBa_I714891 -> 37°C
- for 190-6 and pDS7: 10µl Ampicillin + 10 ml LB-Media + colony of plate
- for eGFP: 13,3 µl Kanamycin + 10 ml LB-Media + 1 colony of plate
-> mixed
- plus: EcoRI (10µg/µl): 0,5 µl resp. PstI (10µg/µl): 0,5 µl
- incubated at room temperature from 12:10 to 15:00, 1 hour at 37°C, 2 hours at 60°C
- frozen at -20°C
- 1 ml of "old" culture + 3 ml LB-Media + 4 µl Kanamycin -> 37°C
- plasmid extraction of pDS7 (458ng/µl), eGFP (55ng/µl), 190-6 (193ng/µl)
-> Protocol: (4 Plasmid extraktion from cells)
- Restriction digest with EcoRI and PstI in buffer H (for testing DNA is correct)
10µg DNA: pDS7 (2µl), eGFP (15µl), 190-6 (10µl)
-> Protocol: (5 Restriction digest)
- Colony for Plasmidextraction (CMV (Kanamycin), eGFP (Kanamycin), pDS7 (Ampicillin), 190-6 (Ampicillin)) plated
- PhiC31o plated on Ampicillin-Agar, stored at 37°C
- 50% Glycerol made (for the glycerolstock PhiC31o)
- CMV (BBa_J52034) from 10.8.2010 inoculated into LB medium with ampicillin, as falsly inoculated in Kanamycin
- Agarosegelelectrophoresis with the digestions (CMV, eGFP, pDS7, 190-6), 125V for 30 minutes and then for 20 minutes;
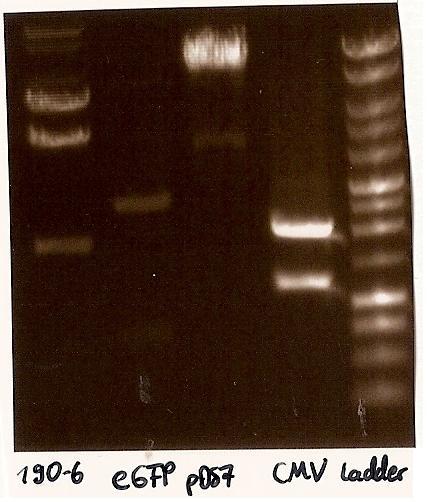
expected DNA bands: 190-6 (4840bp, 1903bp), pDS7 (8027bp, 6bp), CMV (654 bp (Insert), 2079bp (Plasmid)), eGFP (720bp (Insert), 2750bp (Plasmid))
- Correct DNA bands for 190-6 (~4800bp, ~1900bp, ~6700bp (undigested plasmid)) and eGFP (~2000bp (Plasmid), ~750 bp (Insert)); CMV probably not digested (two bands; one probably normal, one supercoiled) and pDS7 not clear
- Restriction digest from CMV (EcoR1, Pst1; 6µl DNA, buffer H) and pDS7 (EcoR1, Spe1; 2µl DNA, buffer B)
Expected DNA bands: CMV see above, pDS7 (3647bp, 3369bp, 1011bp, 6bp)
-> Protocol (5 Restriction digest)
- Agarosegelelectorphoresis for 30 minutes, 150V
- false DNA bands CMV (~1200 bp, ~2000 bp) and pDS7 (~8000bp two bands, ~1100 bp); required to isolate a new colony for these two Plasmidextractions
- Plated the colony from CMV (BBa_J52034) for Plasmidextraction (Ampicillin), as falsly plated on Kanamycin
- transfer PhiC31o culture to new LB medium + Amp, 37°C
- pick up CMV and pDS7 colonies from plates and transfer to LB medium+Amp, 37°C
text
text
text
Next iGEM meeting at 6pm!
Workshop with Tanya on Wiki/Web page at 6pm
text
text
text


![]()
![]()







![]()
![]()

![]()
Apoptosis Notebook
Contents
8-02-2010
8-03-2010
- test5
8-04-2010
header 1
header 2
header 3
row 1, cell 1
row 1, cell 2
row 1, cell 3
row 2, cell 1
row 2, cell 2
row 2, cell 3
8-05-2010
H2Oddes
10,3 µl
RE10 + Buffer H
2,0 µl
acetylated BSA
0,2 µl
DNA
6,0 µl
apple banana peaches
green yellow red
8-06-2010
8-07-2010
8-08-2010
test
8-09-2010
farbnummern für farbige schrift: http://html.nicole-wellinger.ch/hilfen/farbenverzeichnis.html
8-10-2010
Plasmid Isolation
8-11-2010
Prepared overnight culture of eGFP BBa_I714891
Prepared overnight culture of 190-6 and pDS7 and eGFP (BBa_I714891) in falcons
Restriction digest (Restriktionsverdau) of CMV-Promoter BBa_J52034 with EcoRI and PstI
H2Oddest, sterile
10,3 µl
RE10 + Buffer H
2,0 µl
acetylated BSA (18ng/µl)
0,2 µl
DNA (0,143µg/µl)
6,0 µl
Prepare new/fresh overnight culture of CMV-Promoter Biobrick: BBa_J52034
8-12-2010
8-13-2010
8-14-2010
8-15-2010
8-16-2010
8-17-2010
8-18-2010
8-19-2010
8-20-2010
8-21-2010
8-22-2010
![]()
![]()

 "
"