Team:Cambridge/Notebook/Week4
From 2010.igem.org
(Difference between revisions)
(→Sunday) |
|||
| Line 20: | Line 20: | ||
** Organising sequences for synthesis | ** Organising sequences for synthesis | ||
| - | + | {{Team:Cambridge/Templates/Day|Day=Wednesday}} | |
| - | + | {{:Team:Cambridge/Templates/rightpic|src=Cambridge-Glow.jpg}} | |
| + | |||
*Hannah and Theo tested previous day's preparations for competence | *Hannah and Theo tested previous day's preparations for competence | ||
*Peter and Paul transformed phK555 with phK724, placed cells in 30C incubator (above rotating incubator) | *Peter and Paul transformed phK555 with phK724, placed cells in 30C incubator (above rotating incubator) | ||
| Line 27: | Line 28: | ||
**Decided that we may have used wrong technique, but DNA samples in fridge | **Decided that we may have used wrong technique, but DNA samples in fridge | ||
| - | + | {{Team:Cambridge/Templates/Day|Day=Thursday}} | |
| + | {{:Team:Cambridge/Templates/rightpic|src=Cambridge-Gel.jpg}} | ||
===Results=== | ===Results=== | ||
GM230 hns-205 ::Tn10 TetR (black) - somewhat competent | GM230 hns-205 ::Tn10 TetR (black) - somewhat competent | ||
| Line 54: | Line 56: | ||
# Transform competent TOP10 cells with ligation product | # Transform competent TOP10 cells with ligation product | ||
===What happened=== | ===What happened=== | ||
| - | |||
# In the morning we were a bit disconsolate because there was nothing visible on the plates containing phK724 and phK555, and our transformation tests for the variant hns strains were less successful than we had hoped. | # In the morning we were a bit disconsolate because there was nothing visible on the plates containing phK724 and phK555, and our transformation tests for the variant hns strains were less successful than we had hoped. | ||
#However our mood changed when Jim A told us we could not hope for many transformants with 20ul of strains that were not TOP10. We decided to retest the strains with 100 ul plated out.{{ | #However our mood changed when Jim A told us we could not hope for many transformants with 20ul of strains that were not TOP10. We decided to retest the strains with 100 ul plated out.{{ | ||
Revision as of 16:25, 9 August 2010

Notebook: Week 4
Monday
- Wet work
- Transformed competent cells using plasmid phK724 from Jim Slock (thanks!)
- Grew up E. coli containing phK555 in 5ml LB broth in rotating 37°C incubator overnight
- Dry work
- Finalising sequencing requirements
- Dismissed PPK
- Decided to include Luciola cruciata
- Further Wiki work
- Finalising sequencing requirements
Tuesday
- Wet Work
- Made competent cells of 4 different hns mutants: W3110 hns93-1, BW25113 Δhns::kan, W3110 hns-205::Tn10, GM230 hns-205::Tn10
- Now stored in the -80°C freezer for later use
- Dry Work
- Organising sequences for synthesis
Wednesday
- Hannah and Theo tested previous day's preparations for competence
- Peter and Paul transformed phK555 with phK724, placed cells in 30C incubator (above rotating incubator)
- Bill, Emily, Anja performed miniprep and restriction digest then ligation.
- Decided that we may have used wrong technique, but DNA samples in fridge
Thursday
Results
GM230 hns-205 ::Tn10 TetR (black) - somewhat competent
W3110 hns 93-1 (blue) - no transformants
BW25113 Δ hns ::kan (red) - somewhat
W3110 hns-205::Tn10 TetR - no tansformants
Theo's plan
- Miniprep plasmid DNA from luciferase and backbone transformed TOP10 following Qiagen protocol
- Digest luciferase Biobrick with: XbaI, PstI to produce two fragments:
- P end---old backbone---E--Xend
- Xend--luciferase---S--Pend (desired)
- Digest backbone Biobrick with SpeI, PstI to produce:
- Send--short fragment--Pend
- Pend---backbone--E--X--promoter--RBS--Send (desired)
- Run both digest products on a gel
- Extract suitable lengthed fragments from gel:
- Promoter + backbone should be 2153 bp
- Luciferase should be 1653 bp (its 3426 bp backbone should be discarded)
- Perform ligation
- S and X are compatible so we get
Pend---backbone--E--X--promoter--RBS--SXscar--luciferase---S--Pend - The two Pends will also ligate, producing a circular desired plasmid
- S and X are compatible so we get
- Transform competent TOP10 cells with ligation product
What happened
- In the morning we were a bit disconsolate because there was nothing visible on the plates containing phK724 and phK555, and our transformation tests for the variant hns strains were less successful than we had hoped.
- However our mood changed when Jim A told us we could not hope for many transformants with 20ul of strains that were not TOP10. We decided to retest the strains with 100 ul plated out.{{
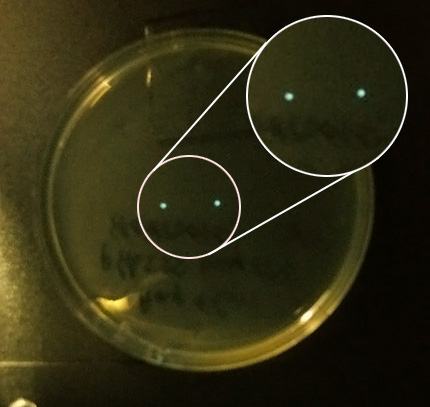
- AND we checked the 30 C incubator again and saw 2 colonies on one of the pHK555 plates, viewed under the camera they were clearly glowing so we gathered in the cold room and passed them around, as our eyes adapted we saw the colonies. We even saw a third glowing colony which we had not spotted on the plate.
Friday
Ligation appeared to have failed. Jim A suggests that we heat to 65 degrees to melt short DNAs after restriction digest, plus that we clean up the DNA with a spin column without gel.
PM
- Hannah and Theo transformed TOP10, red, and black containing phK555 with pHK724 using Jim Slock's EZ Competence buffer
- Anja, Emily drew out various things on petri dishes.
- Set off for trip to lake district
Saturday
Sunday
Went in to the lab on return: Found that:
- TOP10 containing 5/7 (Theo&Hannah, Friday) had been successfully transformed, glowing
- There were some colonies growing on ligation plate
- Logos were mostly dim, except at the edges (weekend incubation too long)
Theo and Peter:
- Redrew logos from *very* turbid liquid culture of pHK555/724
- Prepared overnight cultures of:
- Two hns strains (details?)
- The potential ligations
- The firely luciferase, and backbones, for miniprep if necessary
- + made up the 5mlsish of LB with pHK555/724 (Slock's strain) with 200ml LB
 "
"