Team:ETHZ Basel/Biology
From 2010.igem.org
| Line 14: | Line 14: | ||
== generation of BioBricks == | == generation of BioBricks == | ||
| - | All utilized parts will be generated by PCR and subcloned into the storage vector pSEVA132 (Victor de Lorenzo's lab, KanR, BBR1 ori) allowing blue white screening | + | All utilized parts will be generated by PCR and subcloned into the storage vector pSEVA132 (Victor de Lorenzo's lab, KanR, BBR1 ori) allowing blue white screening: |
| - | + | ||
| - | + | ||
<br>1. Ordering of primers (if template is available) | <br>1. Ordering of primers (if template is available) | ||
<br>2. [[Team:ETHZ_Basel/Lab/protocols#PCR|PCR]] | <br>2. [[Team:ETHZ_Basel/Lab/protocols#PCR|PCR]] | ||
Revision as of 14:26, 6 August 2010
Overview
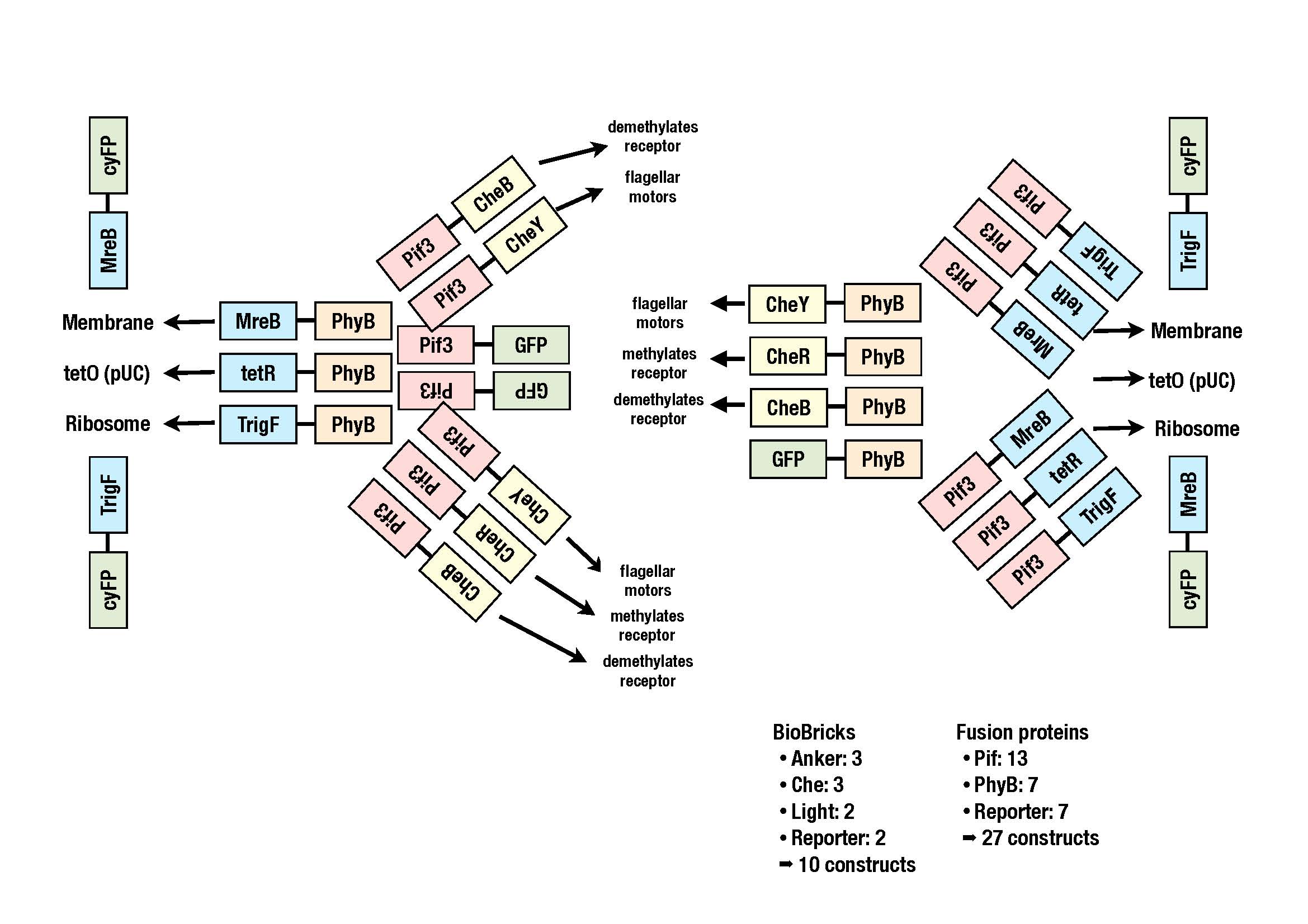
The goal of the iGEM project 2010 of ETH Basel "E.lemming" is to control the tumbling frequency of E. coli. This is achieved by spatially localizing certain elements of the chemotactic network (Che proteins) and thus affecting the activity of their downstream partners.
Reversible localization is achieved by the light-inducible PhyB-PIF3 system detected in plants. PIF3 will be fused to a Che protein and PhyB to a localized anchor within the cell. Red light illumination converts PhyB Pr to Pfr facilitating PhyB-Pif3 interaction and therefore spatially separating the Che protein from its binding partner. Upon far red light stimulus, Pfr converts back to Pr resulting in the dissociation of PhyB-Pif3 and free diffusion of the Che protein.
As modeling of the chemotactic network did not give a clear answer which Che protein should be attacked, several combinations will be investigated.
In view of the chemotactic proteins this includes CheY, CheB and CheR and concerning the localizer MreB (localization to the membrane), tetR (localization to the plasmid via tetO) and trigger factor (localization to the ribosome).
The goal of the wet lab team is to implement this localization system into E. coli.
Cloning Strategy
As we plan to generate several fusion proteins with different linkers, we decided to use the cloning strategy BBF RFC 28: A method for combinatorial multi-part assembly based on the Type IIs restriction enzyme AarI (http://dspace.mit.edu/handle/1721.1/46721). The advantage of this strategy is that we can clone up to 3 different inserts into one vector simultaneously in a 96 well format.
generation of BioBricks
All utilized parts will be generated by PCR and subcloned into the storage vector pSEVA132 (Victor de Lorenzo's lab, KanR, BBR1 ori) allowing blue white screening:
1. Ordering of primers (if template is available)
2. PCR
3. clean-up of PCR product
4. ligation into storage vector
5. Transformation
6. Blue-white screening
7. Sequencing
Due to the presence of rare codons in the sequence of PhyB and Pif3, these two genes will be ordered from GenArt. However, as synthesizing takes several weeks, expression of the wild-type gene of these two proteins will be tested and if satisfying proceeded with these constructs.
generation of biobricks
Currently we are working on putting all the subparts into a storage vector. The image shows all the constructs we plan to clone.
Once the storage vectors are created the acceptor vectors can be generated according to the cloning strategy BBF RFC 28.
testing of biobricks
We will have to test our constructs for the following properties:
- Fusion of che-protein and PhyB or PIF3 for chemotactic functionality
- Fusion of PIF3/PhyB and localizer for proper localization
Chemotactic Functionality
Is our fusion protein still able to maintain its function in the chemotactic pathway? To answer this question we plan to use a swarm test.
Localization
Is our localizer directing PIF3 or PhyB sufficiently to a certain area within the cell? We will use fluorescence microscopy to test for this. To do so we will need an additional fusion protein containing a fluorescent protein. We decided to use GFP and alternatively cyFP because they shouldn’t interfere with our coupling system of PhyB and PIF3 which uses red and far red light. If the fusion between the localizer the fluorescent protein is attracted to a specific site chances are high that a fusion of localizer and PIF3/PhyB also will.
 "
"