Team:Nevada/Notebook
From 2010.igem.org
(Difference between revisions)
(→Team Nevada Notebook) |
(→Team Nevada Notebook) |
||
| Line 202: | Line 202: | ||
* ''Elaine'' | * ''Elaine'' | ||
| - | ** | + | ** Transformed ccdB into NEB, OmniMax, and DEB cells |
** Made LB/KAN plates | ** Made LB/KAN plates | ||
| - | ** Took picture of ccdB Sensitivity Experiment 1 | + | ** Took picture of ccdB Sensitivity Experiment 1 Results |
** GFP Primer Design | ** GFP Primer Design | ||
Revision as of 23:00, 26 July 2010
| You can write a background of your team here. Give us a background of your team, the members, etc. Or tell us more about something of your choosing. | |
| Team Example |
| Home | Team | Official Team Profile | Project | Parts Submitted to the Registry | Modeling | Notebook | Safety |
|---|
Team Nevada Notebook
Week of April 11-17:
- Bryson, Michael, Senny, Tyler
- Made tobacco cell (NT-1) media in Dr. Shintani's lab
Week of April 18-24:
- Bryson
- EcoRI digest of pBIB
- Made Na acetate buffer
Week of April 25-May 1:
- Bryson
- Ran agarose gel of EcoRI digest
Week of May 2-8:
- Elaine
- Ran 0.8% agarose gel of EcoRI digest
- Made LB/KAN plates
- Spread for colonies
- Did miniprep for pBIB liquid cultures
Week of May 9-15:
- Bryson
- Ran 0.8% agarose gel of pBIB post-phenol:chloroform cleanup.
- Did minipreps on additional pBIB liquid cultures.
- Elaine
- Did XbaI and EcoRI digest of pBIB
- Ran 2 0.8% gels of each digest
- Did a miniprep and a Phenol:chloroform clean-up
- Ran 0.8% gel of the XbaI and EcoRI digest with the uncut pBIB
Week of May 16-22:
- Bryson
- Klenow reactions of EcoRI digests
- Phenol:chloroform cleanup of pBIB prior to ligation
- Blunt-end ligation of klenowed pBIB
- Randy Pares and Vidim Gladwell
- Designed primers pBIB-RB-F, pBIB-RB-R, NOS 3'-Foward Nos3' reverse for sequencing the pBIB plant plasmid.
- Elaine
- Made LB/KAN plates
- Made 50mg/ml stock of KAN
- Made 1X TAE buffer
Week of May 23-29:
- Bryson
- Incubated 50 mL liquid culture of E. coli with pBIB (samples 3).
- Miniprepped sample 3, using modified protocol for large/low-copy plasmids.
- EcoRI digest of uncut sample 3
- Prepared 5 mM dNTP stock
- Elaine
- Incubated 50 mL liquid culture of E. coli with pBIB (sample 4 & sample 5)
- Qiaprep miniprep of sample 4 & sample 5 according to manufacturer's modified protocol for large/low-copy plasmids
- Nanodrop of DNA recovery of miniprepped sample 4 & sample 5
- Randy and Vadim
- Sent pBIB vector to Nevada Genomics Center for sequencing with primers designed during the week of May 16-22
- Designed forward and reverse minimal ccdB primers for PCR and sequencing of ccdB gene
- Maxiprepped pBIB vector using QIAGEN QIAfilter Plasmid Maxi Kit
Week of May 30-June 5:
- Bryson
- HinD3 digest of uncut sample 3
- Ran 0.8% gels of samples 1-5 to verify complete digestion by EcoRI
- Pooled uncut samples 3, 4 and 5 (pBIB-pool)
- Recieved 5 µg of pBIB from Randy and Vadim's maxi-prep (pBIB-maxi)
- EcoRI digest of pBIB-pool and pBIB-maxi
- Ran 0.8% agarose gel of EcoRI digests
- Klenow reactions of pBIB-pool and pBIB-maxi
- Made glycerol stocks of pBIB samples 1-5
- Elaine
- EcoRI digest of uncut pBIB sample 4 and 5
- HinD3 digest of uncut sample 4 & sample 5 as a positive control
- Ran 0.8% gels of samples 1-5 to see if EcoRI digest was successful
- Ethanol precipitation of the EcoRI digests of sample 4 & sample 5
- Nanodrop resulted to a low DNA content
- Worked with Bryson for the EcoRI digest of the 5µg of pBIB maxi-prep
- Ran 0.8% agarose gel of the pBIB maxi-prep EcoRI digest
- Modified all protocols of the Binary vector
- Randy and Vadim
- Calculated amount of reagent needed for Deep Vent DNA polymerase reaction (New England Biolabs)
- Programmed thermal cycler for PCR of ccdB gene
- Ran PCR for ccdB
- Prepared LB/KAN Broth
- Gel analyzed resultant ccdB PCR reaction with 1.2% agarose gel
- Reaction was unsuccessful
Week of June 6-12:
- Bryson
- Ligation reactions for pBIB-pool and pBIB-maxi
- Digested pBIB-pool and pBIB-maxi again with EcoRI to linearize any unmodified pBIB
- Transformed Top-10 Cells with modified pBIB (designated pBIB#)
- Obtained two colonies after overnight incubation
- Line-streaked the two colonies (pBIB# 1 and pBIB# 2) on an LB-Kan plate
- Randy and Vadim
- Uploaded pBI101, pBIN19, pBIB-KAN, and ccdB gene to Vector NTI
- Ordered second set of pBIB primers: pBIB-RB-F2, pBIB-RB-F3, pBIB-RB-R2, and pBIB-RB-R3 for sequencing of pBIB
- Modified thermal cycler conditions for PCR of ccdB gene
- Ran PCR for ccdB using HiFi DNA polymerase (Invitrogen)
- Gel analyzed resultant ccdB PCR reaction with 1.2% agarose gel and successfully amplified ccdB gene
- Maxiprepped pBIB vector using QIAGEN QIAfilter Plasmid Maxi Kit
Week of June 13-19:
- Bryson
- Prepared liquid cultures of pBIB# 1 and pBIB# 2
- Miniprepped liquid cultures of pBIB#
- EcoRI and HinDIII digests of pBIB#
- Ran agarose gel of pBIB cut and uncut, pBIB# uncut, pBIB# cut with EcoRI and pBIB# cut with HinDIII to confirm the absence of EcoRI site in pBIB#
- Single-colony streaked pBIB# 2 on a fresh LB-Kan plate
- Elaine
- Worked with Chris to incubate 30 liquid cell cultures
- Ran 0.8% gels of all 30 samples
- Randy and Vadim
- Topocloned ccdB gene into TOPO PCR Blunt II vector
- Determined concentration of pBIB maxipreps using PicoGreen analysis
- Single-colony isolated nine colonies of TOPO-cloned ccdB gene
- Miniprepped cultures of ccdB gene in TOPO-clone
- Digested minipreps using EcoRI
- Ran 1% gel for digested minipreps
- Single colony streaked four cell lines of ccdB gene in TOPO-clone: line 3, line 7, line 8, line 9
- Ordered primers for Left Border Repeat of pBIB: pBIB-LB-F and pBIB-LB-R for sequencing of pBIB vector
Week of June 20-26:
- Bryson
- Selected 3 colonies from pBIB# 2 (2-1, 2-2, 2-3) and streaked on a fresh LB-Kan plate
- Started 20 mL liquid cultures of 2-1, 2-2, and 2-3
- Miniprepped samples
- EcoRI and XbaI digests of samples to confirm that the EcoRI was gone and 2-1, 2-2, 2-3 arose from the same colony
- 0.8% agarose gel of digests
- Randy and Vadim
- Miniprepped isolated cultures of ccdB gene using QIAGEN QIAprep Spin Miniprep Kit
- Nanodropped minipreps
- Digested minipreps using EcoRI
- Ran 1% gel for digested minipreps
- Sent cell lines 7-2 and 8-2 for sequencing to Nevada Genomics Center with ccdB M13 Forward and Reverse primers added
- Analyzed ccdB samples using Vector NTI (Invitrogen)
- Sent cell lines 7-2, 8-2, and 9-1 for sequencing/resequencing to Nevada Genomics Center with ccdB M13 Forward and Reverse primers added
- Elaine
- Primer Design of RD29A
Week of June 27-July 3:
- Randy and Vadim
- Prepared ccdB for MaxiPrep using QIAGEN QIAfilter Plasmid Maxi Kit
- Prepared thermal cycler protocol for pBIB vector
- Performed multiple PCR on pBIB vector
- Transformed ccdB into NEB cells unsuccessfully
- Made chlorophenocol resistant agar plates
- Agarose gel analyzed PCR products
Week of July 4-10:
- Randy and Vadim
- Agarose gel analyzed PCR products
- Transformed ccdB into NEB, OmniMax, and DEB cells
- Made Kanamycin resistant LB plates
- Elaine
- Transformed ccdB into NEB, OmniMax, and DEB cells
- Made LB/KAN plates
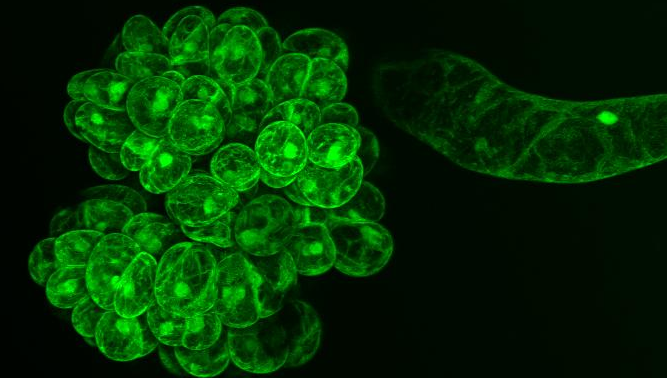
- Took picture of ccdB Sensitivity Experiment 1 Results
- GFP Primer Design
Week of July 11-17:
- Elaine
- Made LB/Amp plates
Week of July 18-24:
- Elaine
- PCR of GFP
- Ran agarose gel analysis of GFP PCR product
Week of July 25-31:
Week of August 1-7:
Week of August 8-14:
Week of August 15-21:
Week of August 22-28:
 "
"