Team:Korea U Seoul/Notebook
From 2010.igem.org
(→[ Digestion] 2010-09-17) |
(→[Discussion] 2010-08-02 ~ 2010-08-29) |
||
| Line 57: | Line 57: | ||
|- | |- | ||
|''mAAA(SpeI)_R'' | |''mAAA(SpeI)_R'' | ||
| - | | | + | |GGACTAGTTTATCACAGGGGCCGTCCG|- |
| - | |- | + | |
|''PzntA(XbaI)_F'' | |''PzntA(XbaI)_F'' | ||
| - | | | + | |GCTCTAGACGTCCGCTCGCTGTATCTC|- |
| - | |- | + | |
|''RFP(PstI)_R'' | |''RFP(PstI)_R'' | ||
| - | | | + | |AACTGCAGCGGCCGCTACTAGTTTATTAAGCACCGGTGGAGTGA|- |
| - | |- | + | |
|''ParsR(XbaI)_F'' | |''ParsR(XbaI)_F'' | ||
| - | | | + | |GCTCTAGACCAACTCAAAATTCACACCTATTAC|- |
| - | |- | + | |
|''GFP(PstI)_R'' | |''GFP(PstI)_R'' | ||
| - | | | + | |AACTGCAGTTAAGGCCTTTTGTATAGTTCATCC|} |
| - | |} | + | |
<br><br> | <br><br> | ||
Revision as of 01:21, 28 October 2010

Brain storming & Work notes
Click on a date to see notes on the meeting & summary of labwork done on that day.
|
|
|
|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Experimental notes
[Discussion] 2010-08-02 ~ 2010-08-29
1. Strategy and overview of iGEM 2010 experiment
2. Design of primers
| Primer | Sequence ( 5’ → 3’ ) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PyodA(EcoRI)_F | cggccgcttctagagCTTCATATTGCCGACAAAGTACG | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| mAAA(SpeI)_R | - | PzntA(XbaI)_F | - | RFP(PstI)_R | - | ParsR(XbaI)_F | - | GFP(PstI)_R | }
[ Preparation of competent cells ] 2010-09-01 ~ 2010-09-03
2. Preparation of 200mL 2x LB broth, TSS solution and LB plates with ampicillin(100μg/mL) and chloramphenicol(25μg /mL), respectively 3. Inoculation of subcultured E. coli to 200mL 2x LB borth 4. Preparation of competent cells by CSBL laboratory protocol 5. Transformation of pUC19 plasmid(10ng/μL) to competent cells for transformation efficiency check
[ Transformation efficiency ] 2010-09-04
[ Amplification of BioBrick parts : pSB1A2 and pSB1C3 ] 2010-09-05
2. 20uL suspension by autoclaved distilled water 3. 3uL transformation to E. coli DH5α 4. Plating to LB(Amp100), LB(Cm25)
[ Genomic DNA extraction ] 2010-09-06
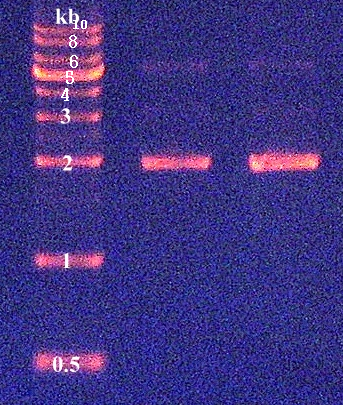
2. E. coli K12 genomic DNA extraction by AccuPrep® Genomic DNA Extraction Kit 3. Confirmation of genomic DNA by agarose gel electrophoresis (Figure 1) 4. Quantification of DNA concentration by NanoDrop : 137.5ng/μL
[ Plasmid DNA extraction : pSB1A3 and pSB1C3 ] 2010-09-07
2. Confirmation of extracted plasmids by agarose gel electrophoresis (Figure 2) 3. Quantification of DNA concentration by NanoDrop
[ PCR : promoters and reporter genes ] 2010-09-13 ~ 2010-09-16
3. Purified PCR products 4. Quantification of DNA concentration by NanoDrop
[ Digestion] 2010-09-171. Digestion of PCR products and pSB1A2
3. Quantification of DNA concentration by NanoDrop
[Chuseok, Korean thanksgiving day] 2010-09-20 ~ 2010-09
[ Ligation & Transformation ] 2010-09-27
[ Confirmation of 1st cloning ] 2010-09-28
2. Inoculation of white colonies to 3mL LB(Amp100)
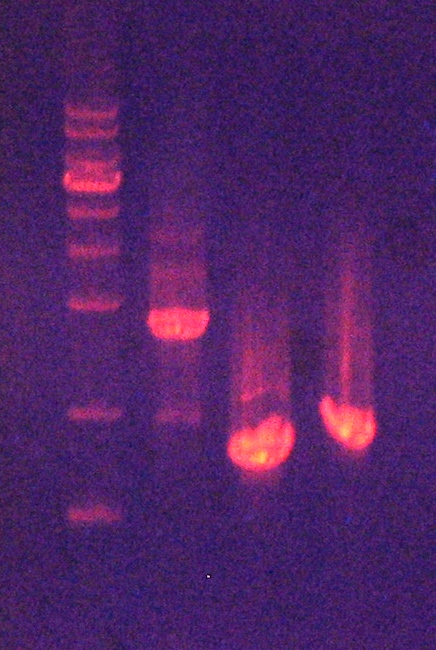
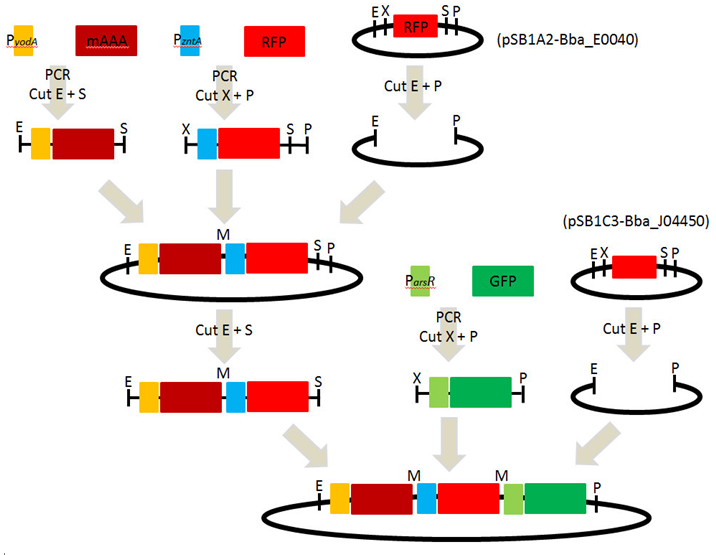
[ Plasmid DNA extraction : pSB1A2-( PyodA-mAAA-PzntA-RFP) ] 2010-09-29
2. Confirmation of extracted plasmids by agarose gel electrophoresis (Figure 5) 3. Recombinant plasmid sequencing by COSMO GeneTech
[ Digestion ] 2010-10-01 ~ 2010-10-03
5. Quantification of DNA concentration by NanoDrop 6. Ligation of each parts : PyodA-mAAA-PzntA-RFP, ParsR-GFP and pSB1C3
[ Confirmation of 2nd cloning ] 2010-10-06
2. Inoculation of white colonies to 3mL LB(Amp100)
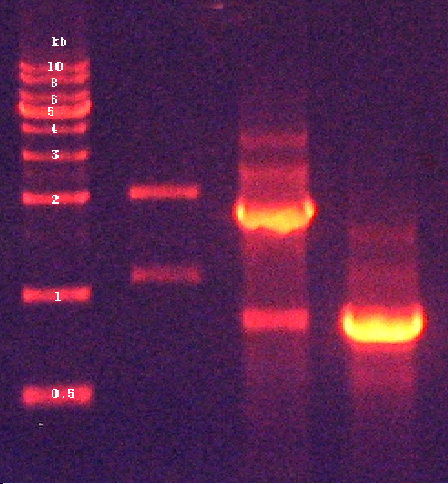
[ Plasmid DNA extraction : pSB1C3-( PyodA-mAAA-PzntA-RFP-ParsR-GFP) ] 2010-10-07
2. Confirmation of extracted plasmids by agarose gel electrophoresis (Figure 7) 3. Recombinant plasmid full-sequencing by COSMO GeneTech
[ Completion : Heavy-metal detector ] 2010-10-18
2. Selection of correct clones 3. Transformation to E. coli BL21(DE3) for expression test
|
 "
"