Team:HokkaidoU Japan/Projects
From 2010.igem.org
(→Detail information about SPI-2 region and SPI-2 carrying E.coli strain) |
(→Detail information about SPI-2 region and SPI-2 carrying E.coli strain) |
||
| Line 27: | Line 27: | ||
==Detail information about SPI-2 region and SPI-2 carrying E.coli strain== | ==Detail information about SPI-2 region and SPI-2 carrying E.coli strain== | ||
| - | Here we show the map of SPI-2 region.(Fig.3) The length of SPI-2 region about 40kb and T3 secretion apparatus is a complex consisted of more than 20 kinds of proteins. As mentioned before cloned SPI-2 on R995 vector was functional in E.coli(K-12).(9) And also it was reported that SPI-2 region cloned on pBeloBAC11(BAC=Bacteria Artificial Chromosome) is functional.(4) So, we decided to request a E.coli(K-12) strain carrying a pBeloBAC11 vector encoding a genome fragment of Salmonella enterica serovar Typhimurium LT2 which covers the SPI-2 region [B_STM07H21 SGSC4024 1464540~1562427] from Salmonella Genetic Stock Center(SGSC) in University of Calgary, Canada http://people.ucalgary.ca/~kesander/index.html. We confirmed the presence of the cloned SPI-2 region in this strain via PCR analysis(Fig.4) shall reffer to it as "pBAC-SPI-2" from now on. Because of the provision of MTA we cannot register this strain as a Bio Brick, but you can also get the same strain from SGSC. | + | Here we show the map of SPI-2 region.(Fig.3) The length of SPI-2 region about 40kb and T3 secretion apparatus is a complex consisted of more than 20 kinds of proteins. As mentioned before cloned SPI-2 on R995 vector was functional in E.coli(K-12).(9) And also it was reported that SPI-2 region cloned on pBeloBAC11(BAC=Bacteria Artificial Chromosome) is functional.(4) So, we decided to request a E.coli(K-12) strain carrying a pBeloBAC11 vector encoding a genome fragment of Salmonella enterica serovar Typhimurium LT2 which covers the SPI-2 region [B_STM07H21 SGSC4024 1464540~1562427] from Salmonella Genetic Stock Center(SGSC) in University of Calgary, Canada [http://people.ucalgary.ca/~kesander/index.html http://people.ucalgary.ca/~kesander/index.html]. We confirmed the presence of the cloned SPI-2 region in this strain via PCR analysis(Fig.4) shall reffer to it as "pBAC-SPI-2" from now on. Because of the provision of MTA we cannot register this strain as a Bio Brick, but you can also get the same strain from SGSC. |
==Construction of E.coli base GFP injector== | ==Construction of E.coli base GFP injector== | ||
Revision as of 16:40, 27 October 2010
Dr. E. coli: The smallest injector in the world
Structure of Type III secretion apparatus
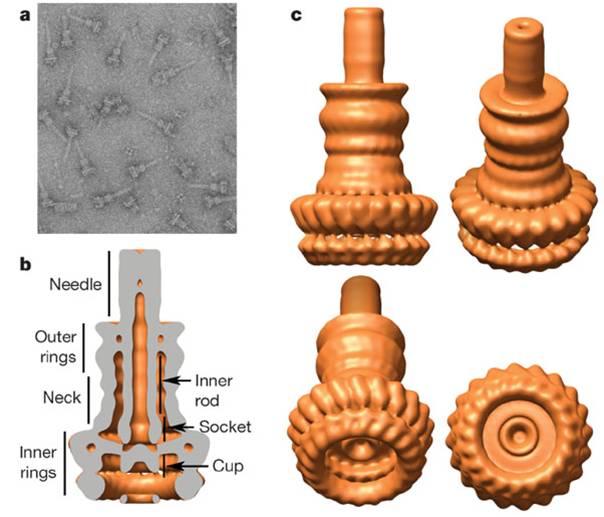
Type III secretion apparatus have a syringe like structure. It can be visible under electro microscope.(2) Its length is about 80nm and the diameter of its needle channel is about 20Å.(3) The length is about 1/10 and the diameter is about 1/400 of an E.coli cell’s minor axis. Thus this is the smallest injector in the world. (Fig.1 a~c)
How does it function?
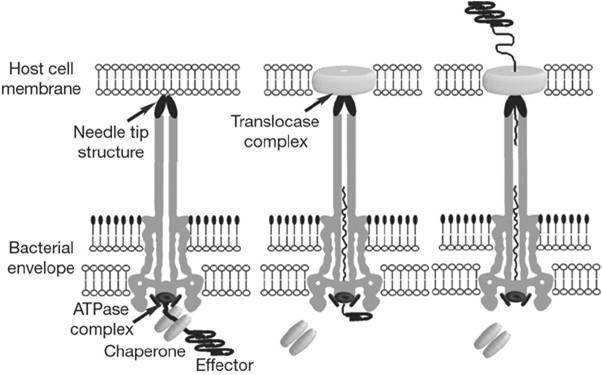
When the needle tip attaches to the host cell membrane a translocator complex that is also secreted by the T3SS is assembled on the host cell membrane and mediates the passage of the effector proteins through the target cell membrane. On the other hand an effector protein, which have a unique T3SS secretion signal domain on its N-terminal, is recognized by the specific chaperone and form an effector-chaperone complex. The secretion machinery, including a T3-secretion-associated ATPase, recognizes the complex. Then, the ATPase stripes the chaperone from the complex, which remains within the bacterial cell, and mediates the unfolding and threading of the effector protein through the central channel of the needle complex. Finally, the translocated effectors re-fold within the host cell to carry out their function. (Fig.2)
Motivation
It is valuable to develop a system that can modulate cells’ behavior impersistently by injecting a desired “protein” directly into cells using a non-pathogenic strain. This system can be applied for many ways. For example, – Inject p53 to kill cancer cells selectively – Inject Yamanaka factors to induce iPS cells And fortunately It was reported in 2007 that T3SS encoded in Salmonella Pathogenesity Island 2(SPI2) is functional in vitro on the E.coli(K-12) strain.(9) However, the T3SS encoded in SPI-2 naturally function inside of the phagosome of the target cell.(8) So, there was no report about whether the SPI-2 T3SS, that is cloned on E.coli(K-12), can inject a heterologous protein from outside of the target cell or not. That is why we have decided to put this challenging project into practice.
Objectives of iGEM 2010
Though it is so exciting to create a cancer killer or an iPS generator E.coli, it is too difficult to aim these practical goals from the very first because it was reported that some domains are not able to pass the needle of T3SS.(1,3) So, we have established four objectives of iGEM 2010 as following.
- To make T3SS available for E.coli
- To inject desired proteins into target eukaryotic cells
- To determine whether the injected protein function correctly
- To deliver the injected protein toward desired compartment in the target cell
Detail information about SPI-2 region and SPI-2 carrying E.coli strain
Here we show the map of SPI-2 region.(Fig.3) The length of SPI-2 region about 40kb and T3 secretion apparatus is a complex consisted of more than 20 kinds of proteins. As mentioned before cloned SPI-2 on R995 vector was functional in E.coli(K-12).(9) And also it was reported that SPI-2 region cloned on pBeloBAC11(BAC=Bacteria Artificial Chromosome) is functional.(4) So, we decided to request a E.coli(K-12) strain carrying a pBeloBAC11 vector encoding a genome fragment of Salmonella enterica serovar Typhimurium LT2 which covers the SPI-2 region [B_STM07H21 SGSC4024 1464540~1562427] from Salmonella Genetic Stock Center(SGSC) in University of Calgary, Canada [http://people.ucalgary.ca/~kesander/index.html http://people.ucalgary.ca/~kesander/index.html]. We confirmed the presence of the cloned SPI-2 region in this strain via PCR analysis(Fig.4) shall reffer to it as "pBAC-SPI-2" from now on. Because of the provision of MTA we cannot register this strain as a Bio Brick, but you can also get the same strain from SGSC.
Construction of E.coli base GFP injector
We have made a T3SS test construct on the tetracycline resistant plasmid[pSB1T3] and transform it to the E.coli(K-12) carrying pBAC-SPI-2 as shown in (Fig.4). It was reported that N-terminus 191 amino acid residues of SlrP(one of the natural effector protein of SPI-2 encoded T3SS) function as a T3 secretion signal domain in Salmonella.(6) And it was also reported that GFP can be secreted through T3SS of Yersinia.(5) So, we fused the N-terminus 191 a.a. of SlrP with the N-terminal of GFP. In addition we located triple NLS(Nuclear Localization Signal) repeats between the T3 secretion signal and GFP so that the injected GFP would localize inside of the nucleus in the target cell. This [T3signal-NLS-GFP] fusion gene is under control of inducible arabinose promoter. In contrast RFP reporter gene, which is joined to the down stream of the GFP construct, is under control of a constitutive promoter. So, if arabinose is added to the culture medium, E.coli produces both GFP and RFP so that the bacteria become yellow. However, if arabinose is not added, only RFP is produced so that the bacteria become red. We have registered the T3SS signal domain mentioned above as a new Bio Brick part.
Bacteria and cell culture condition
To perform the injection assay we used LB medium (1.0% Bacto-Tryptone, 0.5% Bacto-yeast extract, 1.0% NaCl) and magnesium minimal medium (MgM) (11) containing 170 mM MES-NaOH buffer(pH=5.0 or 7.2), 7.5 mM (NH4)2SO4, 5 mM KCl, 1mM KH2PO4, 8uM MgCl2, 38 mM glycerol and 0.1% casamino acids. We named these mediums as MgM5(pH=5.0) and MgM7(pH=7.2). Also we used the acidic cell culture medium RPMI-10%FCS+HCl(pH5.0) [RPMI5] and normal RPMI-10%FCS [RPMI7]. Bacteria were cultured at 37C with aeration and RK13 cells were cultured at 37C in 5.0% CO2. Appropriate antibiotics were added according to the resistance marker on each plasmid.(25 ug/ml of chloramphenicol and 15ug/ml of tetracycline) To induce GFP fusion protein L-arabinose was added to the medium at each step (final concentration = 0.4% ).
Methods of Injection Assay
As mentioned before the T3SS encoded in SPI-2 naturally function inside of the phagosome of the target cell.(8) So, it requires acidic pH to be assembled functionally.(7) However, if the T3 apparatus is assembled successfully under low pH(pH=5.0) condition, only the translocator proteins are secreted through T3SS and effector proteins are not. And it was reported in 2010 that the translocator complex assembles a pore on the phagosome membrane of the host cell enabling the T3SS to sense the neutral pH condition of the cytosol and this pH elevation switches the function of the T3SS to start secretion of effectors.(10) In addition we found that initial growth in MgM7 before the growth in MgM5 improve the production of GFP fusion protein in E.coli.(data not shown) So, at 10h before exposure we transferred E.coli [SPI-2+GFP-T3signal+RFP] overnight culture from LB+arabinose to MgM7+arabinose and grow for 4 hrs. to charge sufficient amount of GFP fusion protein. At 5.5h before exposure bacteria were transferred to MgM5+arabinose and grow for 4 hrs. to assemble T3 secretion apparatus. At 1 hr. before exposure bacteria were washed with RPMI5 three times to remove toxin secreted from E.coli. Then it was resuspended diluted with RPMI5+arabinmose (final O.D.=0.06 at 600nm). On the other hand RK13 cells were seeded on 6well plate (2x105cells/well) in antibiotics free RPMI7 at 20 hrs. before exposure to the E.coli. When the preparation is completed cell culture medium was replaced with 1ml of the E.coli suspension (O.D.=0.06 at 600nm) and incubate at 37C in 5.0% CO2 to mimic the environment inside of the phagosome. At the same time samples of arabinose(-) E.coli[SPI-2+GFP-T3signal+RFP] and E.coli[SPI-2 only] were prepared for the control condition.
References
- Chen LM, Briones G, Donis RO, Galán JE. 2006. Optimization of the delivery of heterologous proteins by the Salmonella enterica serovar Typhimurium type III secretion system for vaccine development. Infect Immun. Vol.74:5826-5833. [http://www.ncbi.nlm.nih.gov/pubmed/16988261 PubMed]
- Galán JE, Wolf-Watz H. 2006. Protein delivery into eukaryotic cells by type III secretion machines. Nature. Vol.444:567-573. Review. [http://www.ncbi.nlm.nih.gov/pubmed/17136086 PubMed]
- Hansen-Wester I, Chakravortty D, Hensel M. 2004. Functional transfer of Salmonella pathogenicity island 2 to Salmonella bongori and Escherichia coli. Infect Immun. Vol.72:2879-2888. [http://www.ncbi.nlm.nih.gov/pubmed/15102800 PubMed]
- Jacobi CA, Roggenkamp A, Rakin A, Zumbihl R, Leitritz L, Heesemann J. 1998. In vitro and in vivo expression studies of yopE from Yersinia enterocolitica using the gfp reporter gene. Mol Microbiol. Vol.30:865-882. [http://www.ncbi.nlm.nih.gov/pubmed/10094634 PubMed]
- Miao EA, Miller SI. 2000. A conserved amino acid sequence directing intracellular type III secretion by Salmonella typhimurium. Proc Natl Acad Sci U S A. Vol.97:7539-7544. [http://www.ncbi.nlm.nih.gov/pubmed/10861017 PubMed]
- Rappl C, Deiwick J, Hensel M. 2003. Acidic pH is required for the functional assembly of the type III secretion system encoded by Salmonella pathogenicity island 2. FEMS Microbiol Lett. Vol.226:363-372. [http://www.ncbi.nlm.nih.gov/pubmed/14553934 PubMed]
- Waterman SR, Holden DW. 2003. Functions and effectors of the Salmonella pathogenicity island 2 type III secretion system. Cell Microbiol. Vol.5:501-511. Review. [http://www.ncbi.nlm.nih.gov/pubmed/12864810 PubMed]
- Wilson JW, Coleman C, Nickerson CA. 2007. Cloning and transfer of the Salmonella pathogenicity island 2 type III secretion system for studies of a range of gram-negative genera. Appl Environ Microbiol. Vol.73:5911-5918. [http://www.ncbi.nlm.nih.gov/pubmed/17675443 PubMed]
- Yu XJ, McGourty K, Liu M, Unsworth KE, Holden DW. 2010. pH sensing by intracellular Salmonella induces effector translocation. Science. Vol.328:1040-1043. [http://www.ncbi.nlm.nih.gov/pubmed/20395475 PubMed]
- Yu XJ, Liu M, Holden DW. 2004. SsaM and SpiC interact and regulate secretion of Salmonella pathogenicity island 2 type III secretion system effectors and translocators. Mol Microbiol. Vol.54:604-619. [http://www.ncbi.nlm.nih.gov/pubmed/15491354 PubMed]
 "
"