Team:Slovenia/PROJECT/proof/studies
From 2010.igem.org
| Line 70: | Line 70: | ||
<!-- OD TU NAPREJ PIŠI BESEDILO --> | <!-- OD TU NAPREJ PIŠI BESEDILO --> | ||
| + | |||
| + | __TOC__ | ||
<h2>Cloning scheme</h2> | <h2>Cloning scheme</h2> | ||
| Line 87: | Line 89: | ||
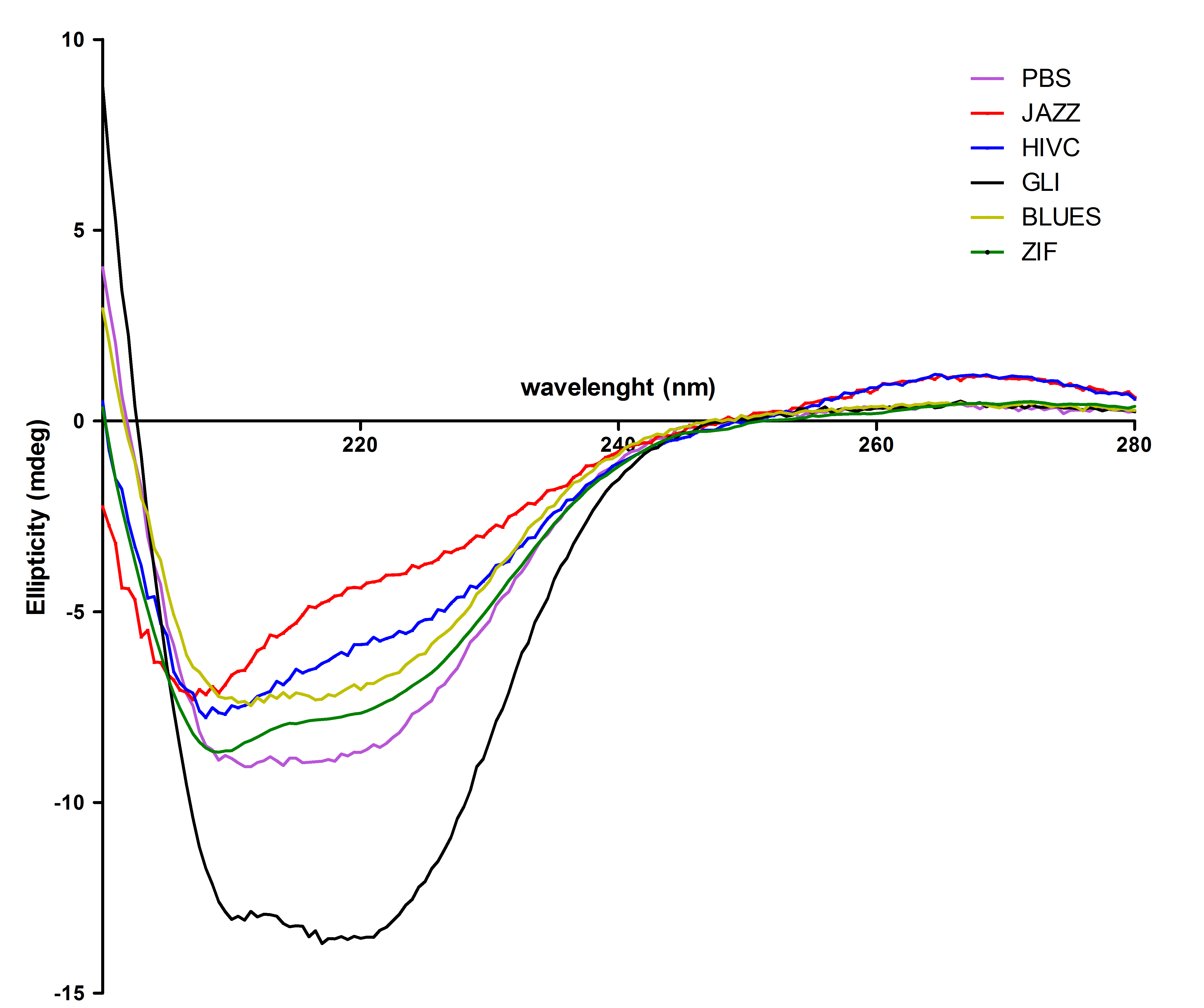
All six proteins were also tested with circular dichorism spectroscopy. Secondary structures were observed successfully: | All six proteins were also tested with circular dichorism spectroscopy. Secondary structures were observed successfully: | ||
| - | [[Image:CDvsiprot(Matej).jpg|thumb|center|700px|'''CD spectroscopy proved our proteins folds secondary structures.''' Secondary structure was determined with CD spectroscopy. From this figure we can see that most of the purified proteins refolded mainly in α-helical structure with small portion of random coil, except Gli wich has mostly α-helical structure.<br><br> | + | [[Image:CDvsiprot(Matej).jpg|thumb|center|700px|'''CD spectroscopy proved our proteins folds secondary structures.''' Secondary structure was determined with CD spectroscopy. From this figure we can see that most of the purified proteins refolded mainly in α-helical structure with small portion of random coil, except Gli wich has mostly α-helical structure.]]<br><br> |
| + | |||
<!--STOP BESEDILO--> | <!--STOP BESEDILO--> | ||
Revision as of 16:09, 27 October 2010
Contents |
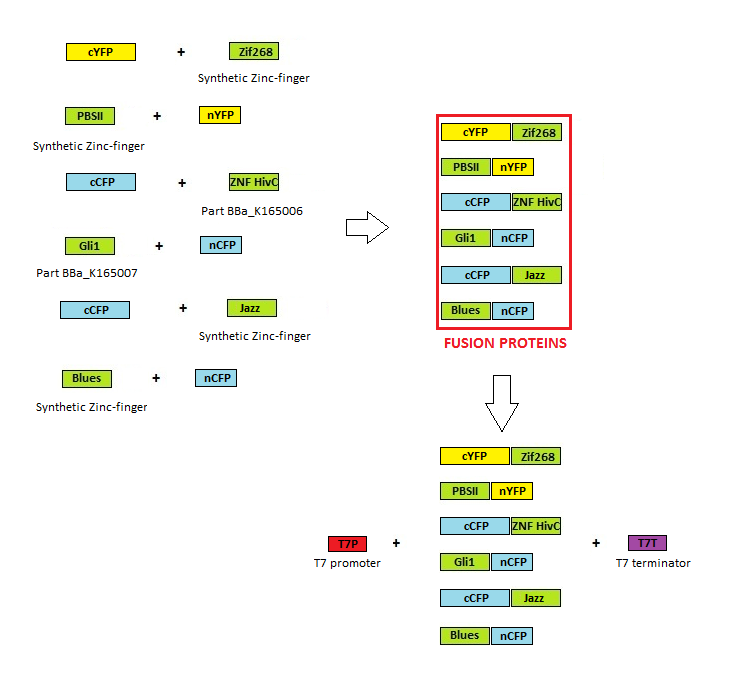
Cloning scheme
Before any in vitro characterisation, DNA-binding proteins had to be obtained. Zinc fingers were firstly tri-point ligated with a particular split GFP and T7 promoter and T7 terminator sequence were added afterwards. T7 promoter enables high production of proteins in E. coli BL21(DE3)pLysS production strain. Zinc fingers were then purified using His tags and used in subsequent experiments. Parts used for production were: BBa_323001, BBa_323015, BBa_323069, BBa_323058, BBa_323004, BBa_323070.
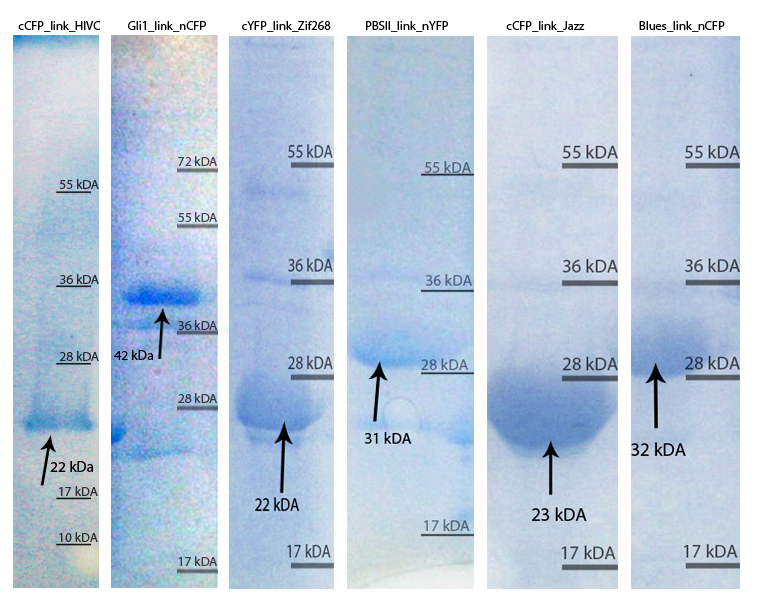
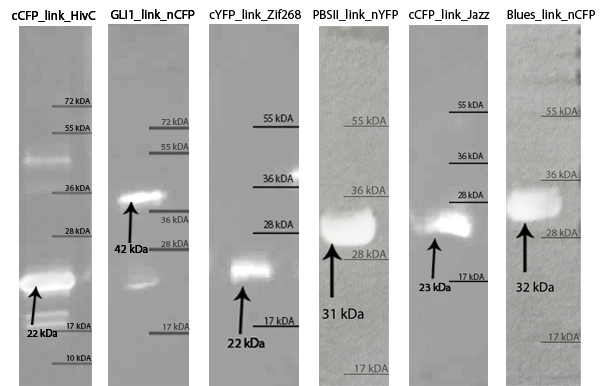
Protein production
Zinc fingers were produced in E.coli BL21(DE3)pLysS and purified with Ni-NTA column according to our protocols in Methods & Parts. Their isolation and purity was confirmed with SDS-page and Western blot techniques. Secondary structure of proteins was determined with circular dichroism. Purified proteins were further used for electrophoretic mobility shift assay (EMSA) test and surface plasmon resonance (SPR) experiments.
All six proteins were also tested with circular dichorism spectroscopy. Secondary structures were observed successfully:
 "
"