Team:Kyoto/Project/Goal C
From 2010.igem.org
Okadakazuya (Talk | contribs) (→Bacterial strains) |
Okadakazuya (Talk | contribs) (→Bacterial strains) |
||
| Line 14: | Line 14: | ||
====Bacterial strains==== | ====Bacterial strains==== | ||
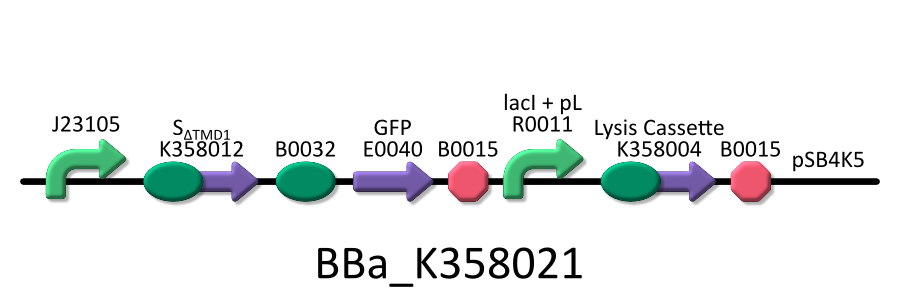
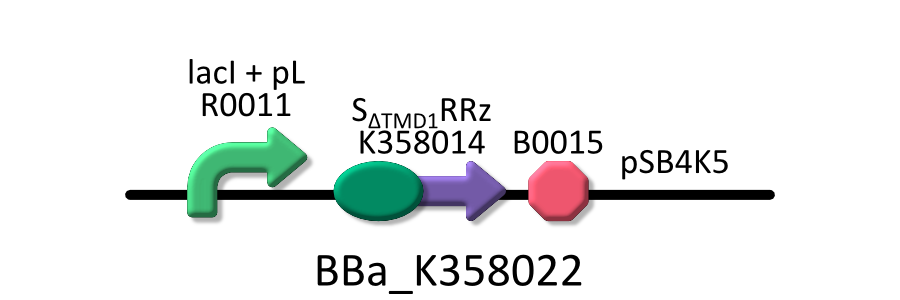
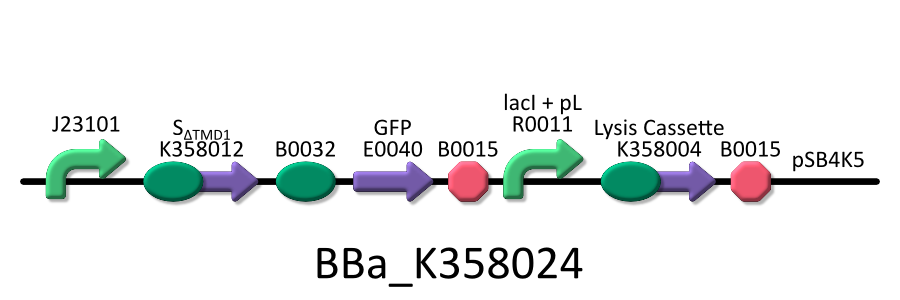
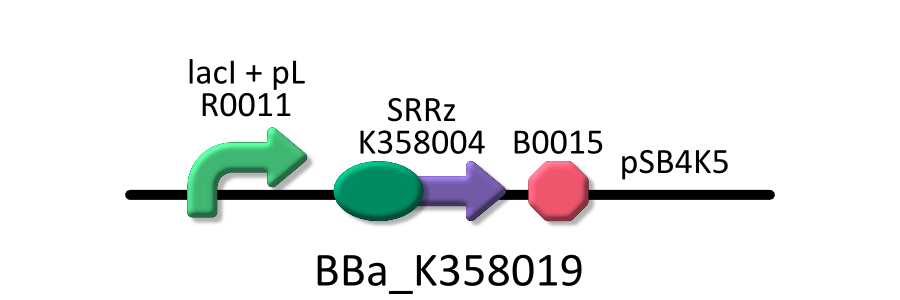
We used four types of E. coli, E. coli KRX transformed with <partinfo>BBa_K358021</partinfo>, KRX transformed with <partinfo>BBa_K358022</partinfo> KRX transformed with <partinfo>BBa_K358024</partinfo> and KPX transfomed with <partinfo>BBa_K358019</partinfo>. | We used four types of E. coli, E. coli KRX transformed with <partinfo>BBa_K358021</partinfo>, KRX transformed with <partinfo>BBa_K358022</partinfo> KRX transformed with <partinfo>BBa_K358024</partinfo> and KPX transfomed with <partinfo>BBa_K358019</partinfo>. | ||
| - | After this, We call each E.coli to abbreviate in order of name, LB2-1, lacLΔ, LB2-2 and lacL . | + | After this, We call each E.coli to abbreviate in order of name, LB2-1, lacLΔ, LB2-2 and lacL. |
====Construct==== | ====Construct==== | ||
Revision as of 13:55, 27 October 2010
Contents |
Goal C: Characterization of the anti-killer gene
Introduction
We selected SΔTMD1 as anti-killer gene of Lysis box. Lysis cassette encodes S gene, R gene and so on and the transmembrane domein 1(TMD1) of this S gene is essential for the function of lysis cassette as killer-gene. So SΔTMD1, which is a S mutant with TMD1 deleted, dominant-negatively inhibits Lysis cassette.(check Learn more) That is why we selected SΔTMD1 as anti-killer gene.
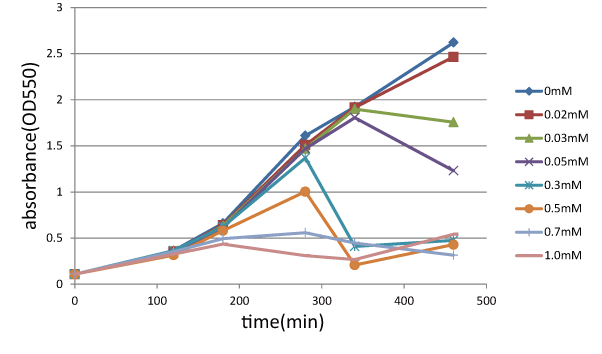
Then, we checked if SΔTMD1 prevent Lysis cassette from causing the cell death. To research this function of SΔTMD1, we used the E.coli transformed with both these genes( <partinfo>BBa_K358021</partinfo>). Because E.coli is immediately dead if killer-gene is constitutively expressed, we made lysis cassette regulated by lac promoter. The E.coli were grown in the medium without IPTG, and after there were enough E.coli, they were cultured in the one with IPTG to express Lysis cassette. After that, we checked, by measuring the absorbance (A550), if E.coli were alive due to anti-killer gene, SΔTMD1 expressed constitutively.
Method
Bacterial strains
We used four types of E. coli, E. coli KRX transformed with <partinfo>BBa_K358021</partinfo>, KRX transformed with <partinfo>BBa_K358022</partinfo> KRX transformed with <partinfo>BBa_K358024</partinfo> and KPX transfomed with <partinfo>BBa_K358019</partinfo>. After this, We call each E.coli to abbreviate in order of name, LB2-1, lacLΔ, LB2-2 and lacL.
Construct
- <partinfo>BBa_K358021</partinfo>:Anti-killergene is induced constitutively and killergene is regulated by lactose promoter.
It was used in experiment 1
- <partinfo>BBa_K358022</partinfo>:lacP + SΔTMD1RRz, TMD1 is deleted from lysis cassette[SRRz].
It was used in experiment 2
- <partinfo>BBa_K358024</partinfo>:This part is the same to BBa_K358021, except for its constitutive promoter[BBa_J23101]. So, this part also induces constitutively SΔTMD1 gene, the anti-killer gene, and lambda lysis cassette[SRRz], the killer gene, is regulated by lacP.
It was used in the additional experiment, which is not finished.
- <partinfo>BBa_K358019</partinfo>:Lysis cassette regulated by lacP, Lysis cassette[SRRz] is regulated by lactose promoter. Activating lactose promoter and expressing SRRz gene, it causes the cell lysis. So, lactose promoter must be repressed when transform this part.
It was used in experiment 2
Mesurement
Experiment 1
We pick up three colonies from each plate, and cultivate them in the supplemented M9 medium overnight, about 16 hours. The overnight cultures were diluted to 0.1~0.11 in pre-warmed fresh the supplemented M9 medium. we measure A550 of the culture containing various IPTG at about 100 minute interval.
Experiment 2
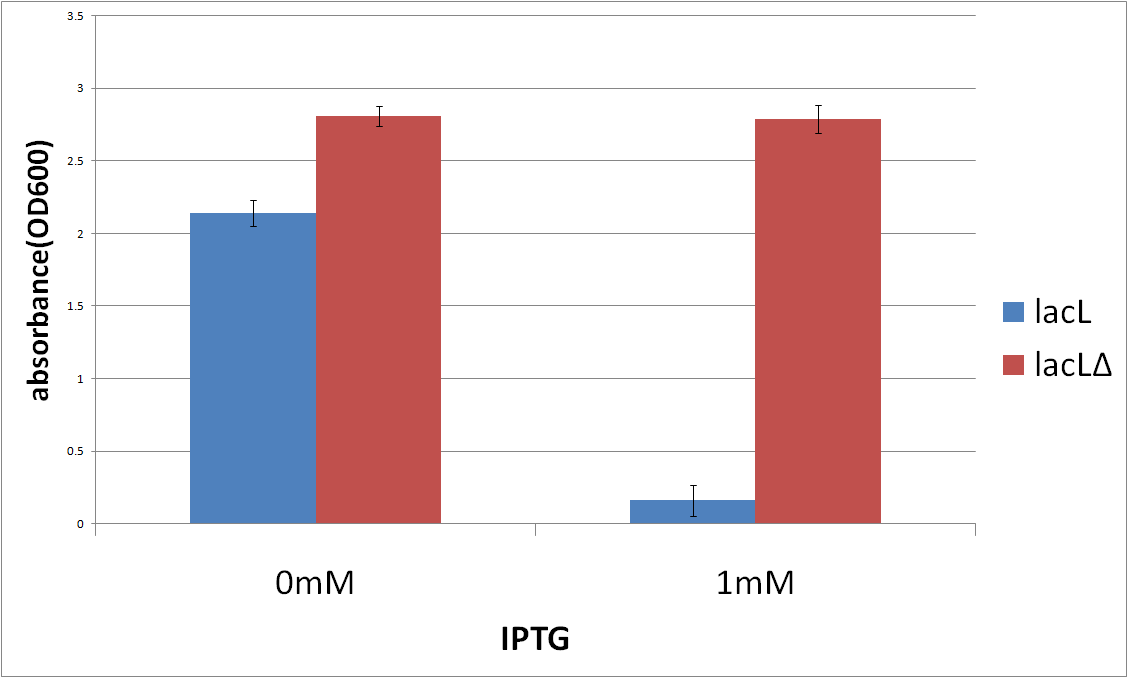
We pick up three colonies from each plate, and cultivate them in the supplemented M9 medium for a night, overnight. The overnight cultures were diluted to 0.1~0.11mM in pre-warmed fresh the supplemented M9 medium. 18h later, we measure A550 of the culture containing 0mM or 1mM IPTG.
Result
Experiment 1: Function check of SΔTMD1
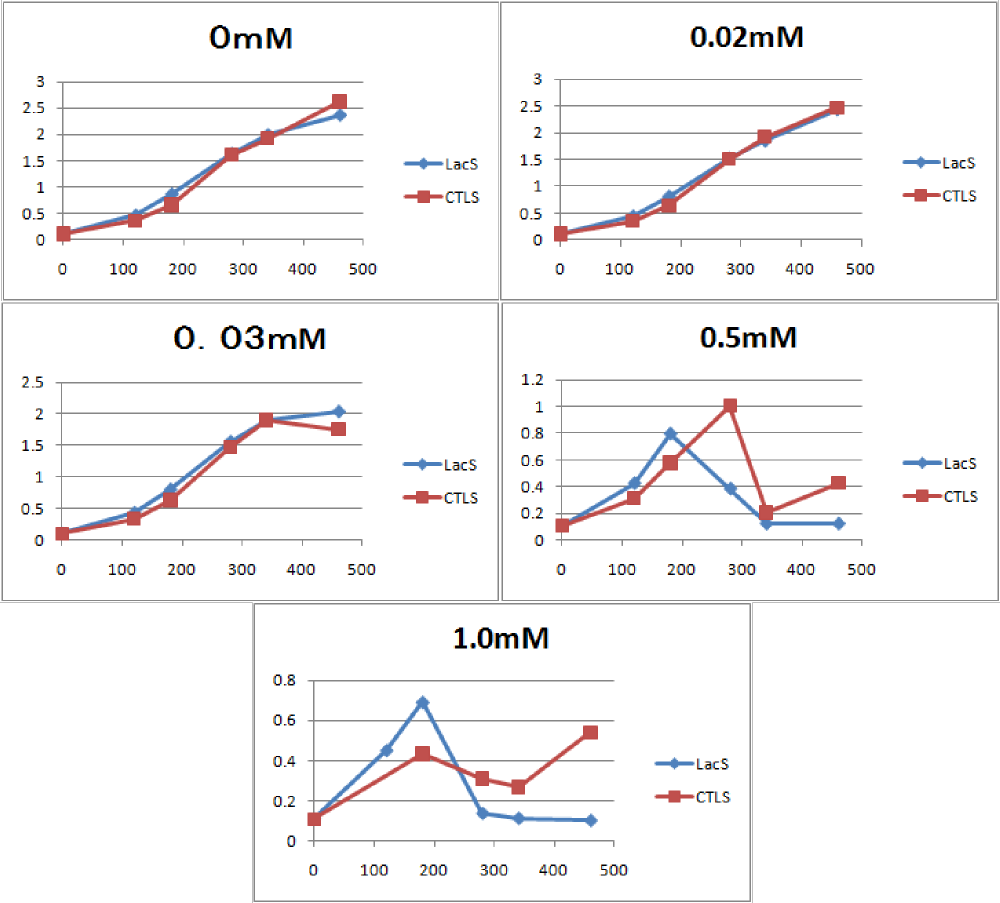
We measured the cell number of E.coli transformed with <partinfo>BBa_K358021</partinfo> at several time after induction by adding several concentration IPTG. The result is below(Fig.1). This result showed that in the medium with more than 0.03mM IPTG, the number of E. coli is decreasing at a certain point. It demonstrated that In at least more than 0.03mM IPTG, SΔTMD1 can't inhibit lysis cassette completly. Then, we measured the cell number of E.coli transformed with <partinfo>BBa_K358019</partinfo> in the same way, and compared the result of E.coli transformed with <partinfo>BBa_K358021</partinfo> with that of E.coli transformed with (<partinfo>BBa_K358019</partinfo>) to check how SΔTMD1 inhibited lysis cassette(Fig.2). In Fig.2, LacS is E.coli transformed with <partinfo>BBa_K358019</partinfo>, CTLS is the one transformed with <partinfo>BBa_K358021</partinfo>. From this result, we knew in more than 0.5mM IPTG both Lacs and CTLA were decreasing in the number at a certain point, or CTLA was a little delayed decreaing in the number. This demonstrated that SΔTMD1 can't correctly inhibit lysis cassette. So, we doubted whether SΔTMD1 really functioned as anti-killer gene. Although SΔTMD1 has no TMD1 of S gene, SΔTMD1 couldn't show the function as anti-killer gene.
Experiment 2:
Then, in order to check if TMD1 of S gene is essential for this killer gene, we measured the cell number of E.coli transformed with Lysis cassette ΔTMD1, which does not have TMD1 of S gene and that is, encodes also SΔTMD1<partinfo>BBa_K358021</partinfo>, This result is below(Fig.3). From the result, we knew if Lysis cassette ΔTMD1 is induced, the cell death was not caused. It proved that S&Delta ;TMD1 cannot function as killer gene and TMD1 is essential for this killer gene, lysis cassette.
Discussion
SΔTMD1 cannot inhibit Lysis cassette completly
These results showed that SΔTMD1 cannot inhibit completely Lysis cassette. This results can't supported a report on lysis (Ref.1) which says that SΔTMD1 without TMD1 dominant-negatively inhibit lysis cassette. Why did we get the result against that report? Probably, there were three reasons below, we thought.
- SΔTMD1 in the plasmid did not worked correctly.
- S&DeltaTMD1 is weaker anti-killer gene than we expected.
- less SΔTMD1 proteins or more lysis cassette proteins was expressed than we expected.
Reason1:SΔTMD1 in the plasmid did not worked correctly.
To make sure the reason, we sequenced BBa_K358021 we used. It’s result showed that this construct is inserted into the plasmid correctly, which demonstrated that SΔTMD1 worked correctly. So, first reason was not right.
Reason2:S&DeltaTMD1 is weaker anti-killer gene than we expected.
As anti-killer gene against lysis cassette, there are two genes, S107 and SΔTMD1(Learn more). S107 is anti-killer gene, but because it has essential TMD1 of S for function of this killer gene, it’s function as anti-killer gene is weak compared with SΔTMD1, which does not have TMD1 of (Learn more). So, we thought that SΔTMD1 is a strong anti-killer gene, but from result of experiment 1 we doubted SΔTMD1 was a strong anti-killer gene. However, I could not make sure the reason.
Reason3:less SΔTMD1 proteins or more lysis cassette proteins was expressed than we expected.
As a report (Ref.2) says, it is necessary for the inhibition of lysis cassette that there are at least 4.73 times more S107, anti-killer gene than lysis cassette proteins. S gene encodes not only S105 but also S107. So SΔTMD1 should have inhibited lysis cassette if at least 4.73 times more SΔTMD1 than lysis cassette were expressed(if reason2 is not right,). Actually, we selected <partinfo>R0011</partinfo> and <partinfo>J23105</partinfo> as each promoter of lysis cassette and SΔTMD1 so that the activity of the promoter of SΔTMD1 is at least five times higher than that of lysis cassette. However, S&Delta TMD1; couldn’t inhibit lysis cassette.
=
Then, in order to make sure the 3rd reason, we changed the promoter of SΔTMD1 to stronger one, and now, are checking the function of SΔTMD1. Surely, this result will help us discuss why SΔTMD1 couldn’t function as we expected. You should expect it!!
 "
"