|
ProteInProgress: a cellular assembly line for protein manufacturing
Implementation and Results: Self-Inducible promoters

Self-inducible promoters
Regulation of signal protein production
Experimental implementation: <partinfo>BBa_K300009</partinfo> part was assembled downstream of different constitutive promoters, thus obtaining a signal molecule generator. The choice of constitutive promoters was performed between the ones belonging to the [http://partsregistry.org/Part:BBa_J23101 Anderson’s promoters collection]; we chose promoters according to their activities reported in the Registry of Standard Biological Parts, in order to have a thick mesh:
| Promoter | Strength (a.u.)
reported in the Registry
|
| <partinfo>BBa_J23100</partinfo> | 2547
|
| <partinfo>BBa_J23101</partinfo> | 1791
|
| <partinfo>BBa_J23105</partinfo> | 623
|
| <partinfo>BBa_J23106</partinfo> | 1185
|
| <partinfo>BBa_J23110</partinfo> | 844
|
| <partinfo>BBa_J23114</partinfo> | 256
|
| <partinfo>BBa_J23116</partinfo> | 396
|
| <partinfo>BBa_J23118</partinfo> | 1429
|
Before constructing the signal generators, <partinfo>BBa_K300009</partinfo> and <partinfo>BBa_K300010</partinfo> under the regulation of one of these constitutive promoters, we evaluated the promoter activities in Relative Promoter Units (R.P.U.) according to Data analysis for RPU evaluation, using the reporter protein RFP (Red Fluorescent Protein) in different experimental conditions (plasmids’ copy number and growth medium), many of them not yet explored and documented:
- high copy number plasmids and LB;
- high copy number plasmids and M9;
- low copy number plasmids and M9.
It was not possible to evaluate promoters activities in low copy number plasmids in LB because the RFP activity was too weak and not distinguishable from the background. RFP fluorescence and Optical Density at 600nm (O.D.600) were measured in 96-well microplates, as reported in Microplate reader experiments for constitutive promoters (R.P.U. evaluation) - Protocol #2 and data were analyzed as reported in Data analysis for RPU evaluation;
Results: results are shown here.
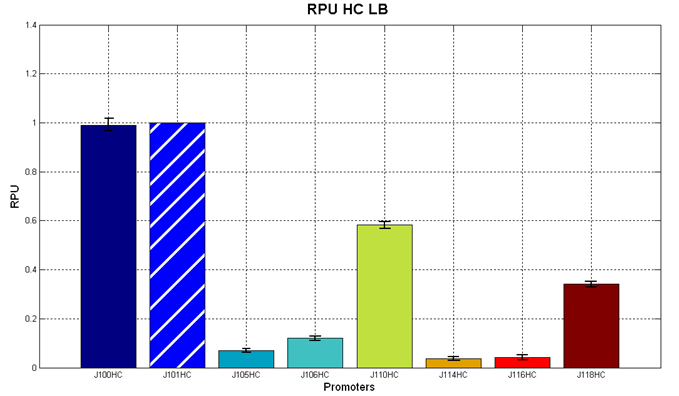 Figure 5 - R.P.U. of the studied promoters from Anderson promoters' collection, LB medium and high copy plasmid (<partinfo>BBa_J61002</partinfo>) | 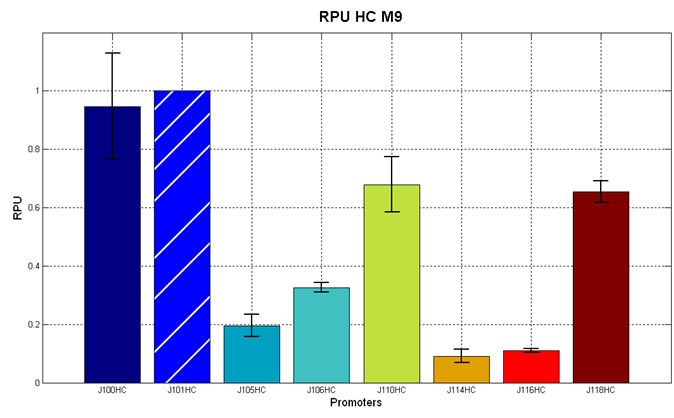 Figure 6 - R.P.U. of the studied promoters from Anderson promoters' collection, M9 medium and high copy plasmid (<partinfo>BBa_J61002</partinfo>) |
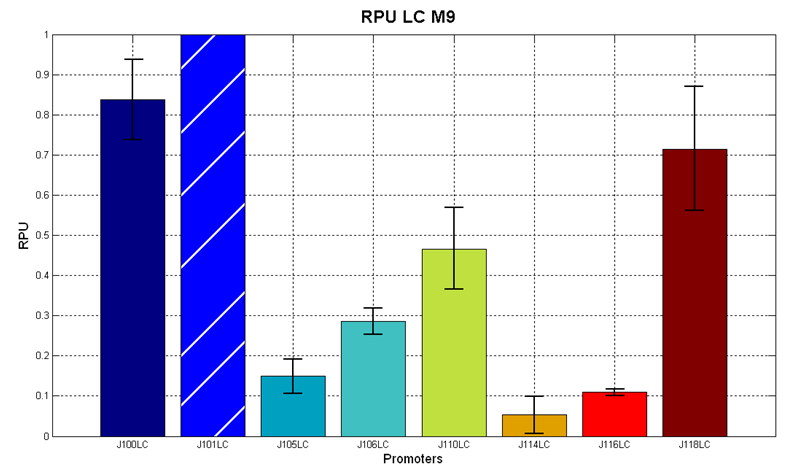 Figure 7 - R.P.U. of the studied promoters from Anderson promoters' collection, M9 medium and low copy plasmid (<partinfo>pSB4C5</partinfo>). These plasmids were constructed by assembling the EcoRI-PstI the <partinfo>BBa_J61002</partinfo>-BBa_J231xx EcoRI-PstI fragment in <partinfo>pSB4C5</partinfo>, in order to transfer the RBS-RFP-TT expression construct from <partinfo>BBa_J61002</partinfo> to <partinfo>pSB4C5</partinfo>. |
Doubling times were evaluated for the described cultures:
| Promoter
| doubling time [minutes]
|
| LB in HC plasmid | M9 in HC plasmid | M9 in LC plasmid
|
| <partinfo>BBa_J23100</partinfo> | | |
|
| <partinfo>BBa_J23101</partinfo> | | |
|
| <partinfo>BBa_J23105</partinfo> | | |
|
| <partinfo>BBa_J23106</partinfo> | | |
|
| <partinfo>BBa_J23110</partinfo> | | |
|
| <partinfo>BBa_J23114</partinfo> | | |
|
| <partinfo>BBa_J23116</partinfo> | | |
|
| <partinfo>BBa_J23118</partinfo> | | |
|
Discussion: we observed that the ranking previously documented in the Registry is not valid in all the conditions, even if a general agreement can be observed. As an example, <partinfo>BBa_J23110</partinfo> in high copy plasmid is stronger than <partinfo>BBa_J23118</partinfo>, in contrast with the ranking reported in the Registry.
After the evaluation of promoter activity, signal generators were constructed in high copy and low copy plasmids: <partinfo>BBa_K300009</partinfo> and <partinfo>BBa_K300010</partinfo> were assembled downstream of the above mentioned promoters, thus obtaining the following parts:
| BioBrick | Description
|
| <partinfo>BBa_K300030</partinfo> | 
J23118
|
| <partinfo>BBa_K300028</partinfo> | 
J23110
|
| <partinfo>BBa_K300029</partinfo> | 
J23116
|
| <partinfo>BBa_K300025</partinfo> | 
J23101
|
| <partinfo>BBa_K300026</partinfo> | 
J23105
|
| <partinfo>BBa_K300027</partinfo> | 
J23106
|
| <partinfo>BBa_K300017</partinfo> | 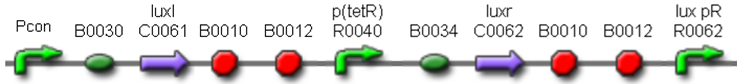
J23118
|
| <partinfo>BBa_K300014</partinfo> | 
J23110
|
| <partinfo>BBa_K300015</partinfo> | 
J23114
|
| <partinfo>BBa_K300016</partinfo> | 
J23116
|
| <partinfo>BBa_K300012</partinfo> | 
J23105
|
Some of the promoters could not be cloned upstream of these devices because they produced LuxI protein amounts that give a high metabolic burden for E. coli, so it was not possible to study all the combinations as transformans could not be obtained in some cases.
For each part, a measurement system was built, exploiting the production of the reporter gene GFP (Green Fluoresent Protein) to evaluate the "switch on" condition of every self-inducible promoter. Many different combinations were explored, in order to provide a library of promoters able to initiate transcription at the desired culture density.
Quantification of the HSL produced
Experimental implementation The new parts were, thus, characterized, measuring the HSL concentration released in the medium after a 6 hour growth of the cultures. All the details are available in this section.
<partinfo>BBa_T9002</partinfo> contained in <partinfo>pSB1A3</partinfo> in E. coli TOP10 was used as a HSL->GFP biosensor. In every experiment, a HSL-GFP calibration curve with known concentration of HSL was produced.
Results The amount of 3OC6-HSL produced after a 6 hours growth by E. coli DH5alpha bearing the parts contained in high copy plasmid <partinfo>pSB1A2</partinfo> is reported in Fig.8 and in the table:
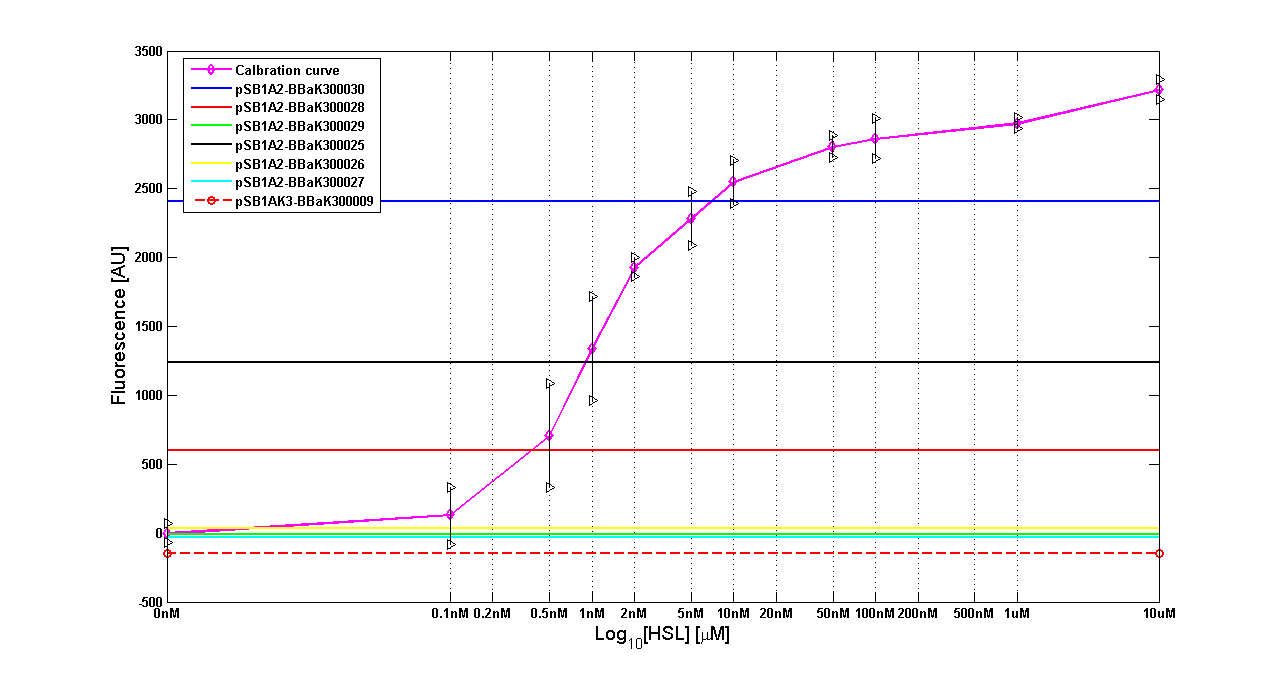 Figure 8 - <partinfo>BBa_T9002</partinfo> calibration curve for detection of [HSL] produced in high copy plasmid |
| BioBrick | Wiki name | E. coli strain | [HSL]
|
| <partinfo>BBa_K300030</partinfo> | I14 | DH5alpha | 0.7 uM
|
| <partinfo>BBa_K300028</partinfo> | I15 | DH5alpha | 0.04 uM
|
| <partinfo>BBa_K300029</partinfo> | I16 | DH5alpha | not detected
|
| <partinfo>BBa_K300025</partinfo> | I17 | DH5alpha | 0.09 uM
|
| <partinfo>BBa_K300026</partinfo> | I18 | DH5alpha | not detected
|
| <partinfo>BBa_K300027</partinfo> | I19 | DH5alpha | 0.002 uM
|
The amount of 3OC6-HSL produced after 6 hours growth by the parts contained in low copy plasmid <partinfo>pSB4C5</partinfo> is reported in Fig.9 and in the table:
 Figure 9 - <partinfo>BBa_T9002</partinfo> calibration curve for detection of [HSL] produced in low copy plasmid |
| BioBrick | Wiki name | E. coli strain | [HSL]
|
| <partinfo>BBa_K300030</partinfo> | I14 | DH5alpha | 0.005 uM
|
| <partinfo>BBa_K300028</partinfo> | I15 | DH5alpha | 0.002 uM
|
| <partinfo>BBa_K300029</partinfo> | I16 | DH5alpha | not detected
|
| <partinfo>BBa_K300025</partinfo> | I17 | DH5alpha | 0.003 uM
|
| <partinfo>BBa_K300026</partinfo> | I18 | DH5alpha | not detected
|
| <partinfo>BBa_K300027</partinfo> | I19 | DH5alpha | not detected
|
Discussion These experiments provided extremely useful informations about the capability of the signal generators to produce the 3OC6-HSL signal molecule. Data are quantitative, but incomplete because for weak promoters or medium-strength promoters contained in a low copy number plasmid the amount of 3OC6-HSL was not detectable using this system. However, this simple experiment shows that there is a strong correlation between the strength of promoter and the amount of signal molecule produced. These results confirm that the production of the autoinducer can be engineered in E. coli and different expression systems reach different amounts of 3OC6-HSL in the growth media as a function of the promoter strength. Thus, these results demonstrate that self-inducible circuits can be rationally designed from a set of well characterized standard parts.
Modulation of plasmid copy number
Signal generator and sensor device were assembled in an unique part (such as <partinfo>BBa_K300017</partinfo>, <partinfo>BBa_K300014</partinfo>, <partinfo>BBa_K300015</partinfo>, <partinfo>BBa_K300016</partinfo> and <partinfo>BBa_K300012</partinfo>) beared on high copy number plasmid <partinfo>pSB1A2</partinfo> or low copy number plasmid <partinfo>pSb4C5</partinfo>. A third alternative was the assembly of signal generator on a low copy number plasmid (<partinfo>pSB4C5</partinfo>) and the receiver device on high copy number plasmid (<partinfo>pSB1A2</partinfo>).
The circuits we obtained and tested are summarized here:
BioBrick
Sender | Description | Sender Vector | <partinfo>BBa_F2620</partinfo>
Receiver vector | BioBrick composite part
|
| <partinfo>BBa_K300030</partinfo>
| 
J23118
| <partinfo>pSB1A2</partinfo>
HC
| <partinfo>BBa_K300017</partinfo>
|
| <partinfo>BBa_K300028</partinfo>
| 
J23110
| <partinfo>pSB1A2</partinfo>
HC
| <partinfo>BBa_K300014</partinfo>
|
| <partinfo>BBa_K300029</partinfo>
| 
J23116
| <partinfo>pSB1A2</partinfo>
HC
| <partinfo>BBa_K300016</partinfo>
|
| <partinfo>BBa_K300026</partinfo>
| 
J23105
| <partinfo>pSB1A2</partinfo>
HC
| <partinfo>BBa_K300012</partinfo>
|
| xxx | 
J23114
| <partinfo>pSB1A2</partinfo>
HC
| <partinfo>BBa_K300015</partinfo>
|
| <partinfo>BBa_K300030</partinfo>
| 
J23118
| <partinfo>pSB4C5</partinfo>
LC
| <partinfo>BBa_K300017</partinfo>
|
| <partinfo>BBa_K300028</partinfo>
| 
J23110
| <partinfo>pSB4C5</partinfo>
LC
| <partinfo>BBa_K300014</partinfo>
|
| <partinfo>BBa_K300029</partinfo>
| 
J23116
| <partinfo>pSB4C5</partinfo>
LC
| <partinfo>BBa_K300016</partinfo>
|
| <partinfo>BBa_K300026</partinfo>
| 
J23105
| <partinfo>pSB4C5</partinfo>
LC
| <partinfo>BBa_K300012</partinfo>
|
| <partinfo>BBa_K300030</partinfo>
| 
J23118
| <partinfo>pSB4C5</partinfo>
LC
| <partinfo>pSB1A2</partinfo>
HC
| Parts are contained in two different vectors
|
| <partinfo>BBa_K300028</partinfo>
| 
J23110
| <partinfo>pSB4C5</partinfo>
LC
| <partinfo>pSB1A2</partinfo>
HC
| Parts are contained in two different vectors
|
| <partinfo>BBa_K300029</partinfo>
| 
J23116
| <partinfo>pSB4C5</partinfo>
LC
| <partinfo>pSB1A2</partinfo>
HC
| Parts are contained in two different vectors
|
| <partinfo>BBa_K300025</partinfo>
| 
J23101
| <partinfo>pSB4C5</partinfo>
LC
| <partinfo>pSB1A2</partinfo>
HC
| Parts are contained in two different vectors
|
| <partinfo>BBa_K300026</partinfo>
| 
J23105
| <partinfo>pSB4C5</partinfo>
LC
| <partinfo>pSB1A2</partinfo>
HC
| Parts are contained in two different vectors
|
| <partinfo>BBa_K300027</partinfo>
| 
J23106
| <partinfo>pSB4C5</partinfo>
LC
| <partinfo>pSB1A2</partinfo>
HC
| Parts are contained in two different vectors
|
Results
The following measurement systems were realized assembling GFP downstream of each self-inducible device. The parts characterized are reported in this table:
| Sender device
| Sensor systems with GFP
| Measurement Device
|
<partinfo>BBa_K300030</partinfo> in <partinfo>pSB1A2</partinfo>

J23118
| <partinfo>BBa_T9002</partinfo> in <partinfo>pSB1A2</partinfo>

| <partinfo>BBa_K300024</partinfo>
in <partinfo>pSB1A2</partinfo>
|
<partinfo>BBa_K300028</partinfo> in <partinfo>pSB1A2</partinfo>

J23110
| <partinfo>BBa_T9002</partinfo> in <partinfo>pSB1A2</partinfo>

| <partinfo>BBa_K300021</partinfo>
in <partinfo>pSB1A2</partinfo>
|
<partinfo>BBa_K300029</partinfo> in <partinfo>pSB1A2</partinfo>

J23116
| <partinfo>BBa_T9002</partinfo> in <partinfo>pSB1A2</partinfo>

| <partinfo>BBa_K300022</partinfo>
in <partinfo>pSB1A2</partinfo>
|
<partinfo>BBa_K300026</partinfo> in <partinfo>pSB1A2</partinfo>

J23105
| <partinfo>BBa_T9002</partinfo> in <partinfo>pSB1A2</partinfo>

| <partinfo>BBa_K300019</partinfo>
in <partinfo>pSB1A2</partinfo>
|
xxx

J23114
| <partinfo>BBa_T9002</partinfo> in <partinfo>pSB1A2</partinfo>

| <partinfo>BBa_K300023</partinfo>
in <partinfo>pSB1A2</partinfo>
|
<partinfo>BBa_K300030</partinfo> in <partinfo>pSB4C5</partinfo>

J23118
| <partinfo>BBa_T9002</partinfo> in <partinfo>pSB4C5</partinfo>

| <partinfo>BBa_K300024</partinfo>
in <partinfo>pSB4C5</partinfo>
|
<partinfo>BBa_K300028</partinfo> in <partinfo>pSB4C5</partinfo>

J23110
| <partinfo>BBa_T9002</partinfo> in <partinfo>pSB4C5</partinfo>

| <partinfo>BBa_K300021</partinfo>
in <partinfo>pSB4C5</partinfo>
|
<partinfo>BBa_K300029</partinfo> in <partinfo>pSB4C5</partinfo>

J23116
| <partinfo>BBa_T9002</partinfo> in <partinfo>pSB4C5</partinfo>

| <partinfo>BBa_K300022</partinfo>
in <partinfo>pSB4C5</partinfo>
|
<partinfo>BBa_K300026</partinfo> in <partinfo>pSB4C5</partinfo>

J23105
| <partinfo>BBa_T9002</partinfo> in <partinfo>pSB4C5</partinfo>

| <partinfo>BBa_K300019</partinfo>
in <partinfo>pSB4C5</partinfo>
|
<partinfo>BBa_K300030</partinfo> in <partinfo>pSB4C5</partinfo>

J23118
| <partinfo>BBa_T9002</partinfo> in <partinfo>pSB1A3</partinfo>

| Sender and Receiver are contained in two different plasmids,
cotransformed in the same cell
|
<partinfo>BBa_K300028</partinfo> in <partinfo>pSB4C5</partinfo>

J23110
| <partinfo>BBa_T9002</partinfo> in <partinfo>pSB1A3</partinfo>

| Sender and Receiver are contained in two different plasmids,
cotransformed in the same cell
|
<partinfo>BBa_K300029</partinfo> in <partinfo>pSB4C5</partinfo>

J23116
| <partinfo>BBa_T9002</partinfo> in <partinfo>pSB1A3</partinfo>

| Sender and Receiver are contained
in two different plasmids,
cotransformed in the same cell
|
<partinfo>BBa_K300026</partinfo> in <partinfo>pSB4C5</partinfo>

J23105
| <partinfo>BBa_T9002</partinfo> in <partinfo>pSB1A3</partinfo>

| Sender and Receiver are contained
in two different plasmids,
cotransformed in the same cell
|
<partinfo>BBa_K300025</partinfo> in <partinfo>pSB4C5</partinfo>

J23101
| <partinfo>BBa_T9002</partinfo> in <partinfo>pSB1A3</partinfo>

| Sender and Receiver are contained
in two different plasmids,
cotransformed in the same cell
|
<partinfo>BBa_K300027</partinfo> in <partinfo>pSB4C5</partinfo>

J23106
| <partinfo>BBa_T9002</partinfo> in <partinfo>pSB1A3</partinfo>

| Sender and Receiver are contained
in two different plasmids,
cotransformed in the same cell
|
Cultures of E. coli TOP10 bearing the plasmids containing the self-inducible devices expressing G.F.P. were grown according to this protocol and all data collected were analyzed as explained in this section
 Growth curve of <partinfo>BBa_K300019</partinfo> (O.D.600) | 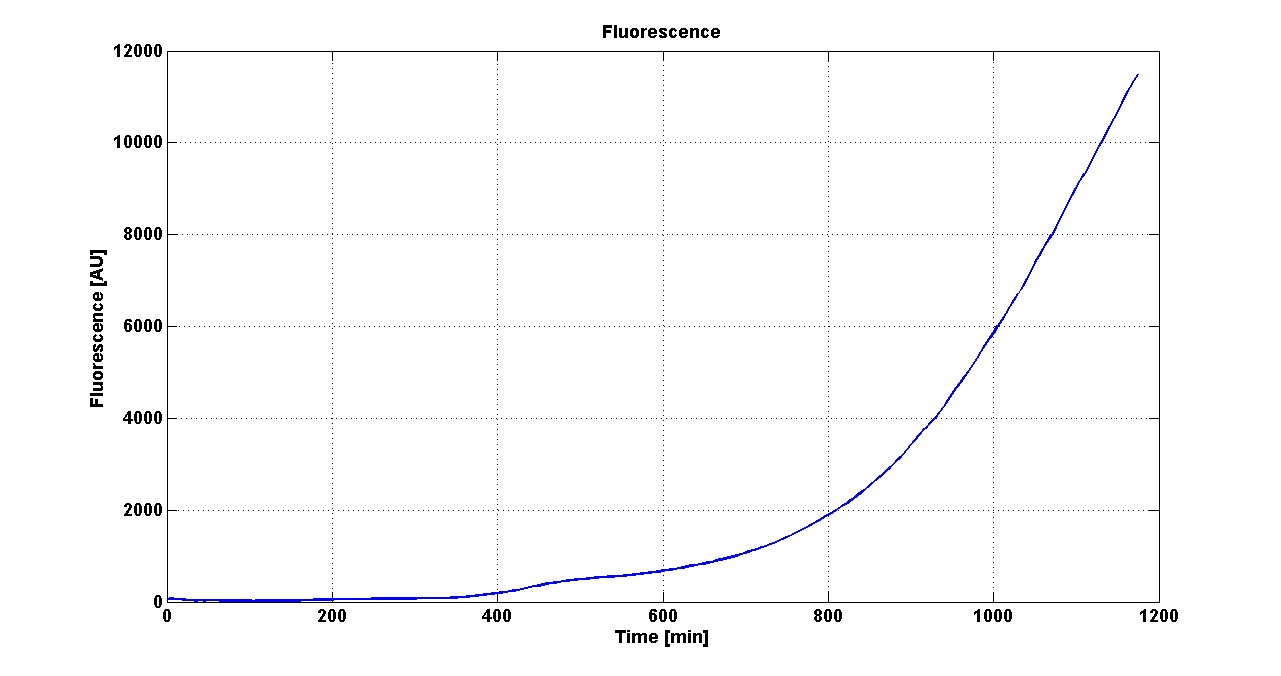 Fluorescence curve of <partinfo>BBa_K300019</partinfo> (G.F.P.) |
 Fluorescence VS Optical density curve of <partinfo>BBa_K300019</partinfo> | 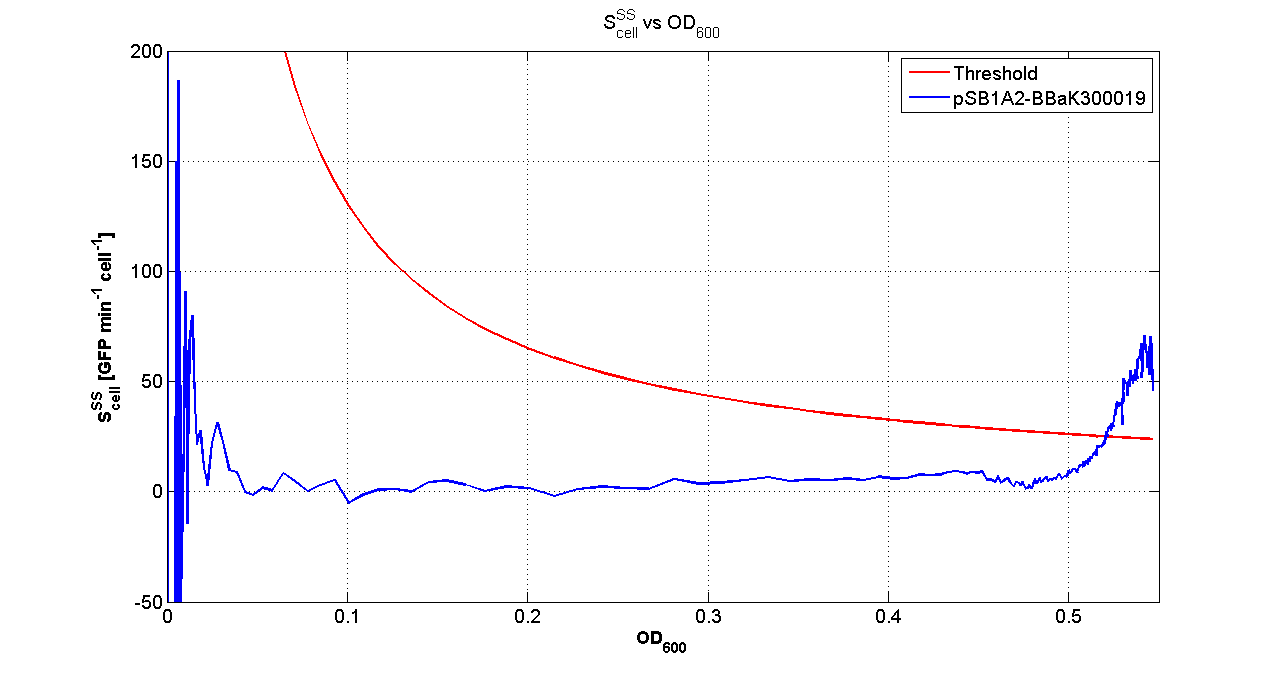 Scell=(dGFP/dt)/O.D.600 and threshold |
Thus, these BioBrick parts can be used to express recombinant proteins without adding an inducer to trigger the transcription of their genes; in large-scale production of such proteins this strategy could be also cost saving.
For every self-inducible device, several parameters were evaluated:
- O.D.start is the O.D.600 corresponding to the transcription initiation of the gene of interest, it was evaluated as reported in this section
- K_HSL is the HSL synthesys rate per cell, it was estimated with the algorithm described here
- Doubling time is the period of time required for a cell population to double. It was evaluated as described in Doubling time evaluation section
- Scell_ratio was evaluated as (Scell_max_Phi)/(Scell_max_J101). Phi is the self-inducible device of ineterst, J101 is the reference standard <partinfo>BBa_J23101</partinfo> contained in the same vector of the receiver device. Scell_max_phi was evaluated for times subsequent to the transcription initiation.
Results are summarized in the following tables:
Tab. 1 - Sender and Receiver on high copy plasmid <partinfo>pSB1A2</partinfo>
Self-inducible device
working
not working
| Description
| LB
| M9
|
| O.D.start
| K_HSL
[nmol/min]
| Doubling time
[min]
| Scell ratio
| O.D.start
| K_HSL
[nmol/min]
| Doubling time
[min]
| Scell ratio
|
| <partinfo>BBa_K300017</partinfo> (wiki name: I7) in <partinfo>pSB1A2</partinfo> plasmid
| 
| Constitutive
| 3.11 10^-16
±
2.23 10^-17
| 31.15
±
2.52
| 0.95
±
0.15
| 0.027
±
0.002
| 1.52 10^-15
±
9.76 10^-17
| 61.28
±
2.67
| 1.68
±
0.23
|
| <partinfo>BBa_K300014</partinfo> (wiki name: I8) in <partinfo>pSB1A2</partinfo> plasmid
| 
| X
| X
| 27.86
±
1.14
| X
| 0.13
±
0.023 **
| 3.59 10^-16
±
1.22 10^-16 **
| 0.37
±
0.13 **
| 53.09
±
1.18 **
|
| <partinfo>BBa_K300015</partinfo> (wiki name: I9) in <partinfo>pSB1A2</partinfo> plasmid
| 
| 0.33
±
*
| 8.94 10^-17
±
*
| 33.81
±
3.03
| 0.98
±
*
| 0.38
±
0.02
| 3.78 10^-17
±
4.65 10^-18
| 57.20
±
1.32
| 0.07
±
0.01
|
| <partinfo>BBa_K300016</partinfo> (wiki name: I10) in <partinfo>pSB1A2</partinfo> plasmid
| 
| 0.54
±
*
| 7.53 10^-18
±
*
| 39.55
±
0.32
| 0.21
±
*
| 0.32
±
0.02
| 5.70 10^-17
±
7.75 10^-18
| 55.33
±
5.19
| 0.13
±
0.02
|
| <partinfo>BBa_K300012</partinfo> (wiki name: I12) in <partinfo>pSB1A2</partinfo> plasmid
| 
| 0.50
±
0.01
| 1.16 10^-17
±
6.41 10^-19
| 36.66
±
2.50
| 0.58
±
0.02
| 0.30
±
0.03
| 6.53 10^-17
±
1.43 10^-17
| 50.92
±
2.92
| 0.21
±
0.06
|
Tab. 2 - Sender and Receiver on low copy plasmid <partinfo>pSB4C5</partinfo>
Self-inducible device
working
not working
| Description
| LB
| M9
|
| O.D.start
| K_HSL
[nmol/min]
| Doubling time
[min]
| Scell ratio
| O.D.start
| K_HSL
[nmol/min]
| Doubling time
[min]
| Scell ratio
|
| <partinfo>BBa_K300017</partinfo> (wiki name: I7) in <partinfo>pSB4C5</partinfo> plasmid
| 
| X
| X
| X
| X
| 0.24
±
0.0004 **
| not computed
| 62.41
±
1.56 **
| not computed
|
| <partinfo>BBa_K300014</partinfo> (wiki name: I8) in <partinfo>pSB4C5</partinfo> plasmid
| 
| X
| X
| X
| X
| X
| X
| X
| X
|
| <partinfo>BBa_K300015</partinfo> (wiki name: I9) in <partinfo>pSB4C5</partinfo> plasmid
| 
| X
| X
| X
| X
| X
| X
| X
| X
|
| <partinfo>BBa_K300016</partinfo> (wiki name: I10) in <partinfo>pSB4C5</partinfo> plasmid
| 
| X
| X
| X
| X
| X
| X
| X
| X
|
| <partinfo>BBa_K300012</partinfo> (wiki name: I12) in <partinfo>pSB4C5</partinfo> plasmid
| 
| X
| X
| X
| X
| X
| X
| X
| X
|
Tab. 3 - Sender on low copy plasmid <partinfo>pSB4C5</partinfo> and Receiver on high copy plasmid <partinfo>pSB1A3</partinfo>
Self-inducible device
working
not working
| Description
| LB
| M9
|
| O.D.start
| K_HSL
[nmol/min]
| Doubling time
[min]
| Scell ratio
| O.D.start
| K_HSL
[nmol/min]
| Doubling time
[min]
| Scell ratio
|
<partinfo>BBa_K300030</partinfo>
(wiki name: I14)
in <partinfo>pSB4C5</partinfo> plasmid
| 
LC
| 
HC
| Constitutive
| 2.92 10^-16
±
8.16 10^-18
| 33.20
±
1.46
| 1.20
±
0.20
| 0.05
±
0.005
| 8.06 10^-16
±
1.28 10^-16
| 58.27
±
5.66
| 0.91
±
0.15
|
<partinfo>BBa_K300028</partinfo>
(wiki name: I15)
in <partinfo>pSB4C5</partinfo> plasmid
| 
LC
| 
HC
| 0.32
±
0.04 **
| 8.31 10^-17
±
3.92 10^-17 **
| 33.24
±
1.27
| 0.61
±
0.04 **
| 0.15
±
0.007
| 1.72 10^-16
±
6.65 10^-18
| 65.57
±
5.99
| 0.26
±
0.03
|
<partinfo>BBa_K300029</partinfo>
(wiki name: I16)
in <partinfo>pSB4C5</partinfo> plasmid
| 
LC
| 
HC
| 0.31
±
*
| 1.17 10^-16
±
*
| 35.46
±
2.85
| 0.57
±
*
| X
| X
| X
| X
|
<partinfo>BBa_K300025</partinfo>
(wiki name: I17)
in <partinfo>pSB4C5</partinfo> plasmid
| 
LC
| 
HC
| Constitutive
| 2.85 10^-16
±
1.90 10^-17
| 33.27
±
2.85
| 1.09
±
0.28
| 0.1
±
0.01
| 3.81 10^-16
±
4.68 10^-17
| 59.02
±
8.28
| 0.45
±
0.08
|
<partinfo>BBa_K300026</partinfo>
(wiki name: I18)
in <partinfo>pSB4C5</partinfo> plasmid
| 
LC
| 
HC
| X
| X
| 34.63
±
1.31
| X
| 0.53
±
0.03 **
| 1.78 10^-17
±
1.36 10^-18 **
| 61.68
±
7.08
| 0.03
±
0.002 **
|
<partinfo>BBa_K300027</partinfo>
(wiki name: I19)
in <partinfo>pSB4C5</partinfo> plasmid
| 
LC
| 
HC
| X
| X
| 33.80
±
1.78
| X
| 0.33
±
0.05
| 5.46 10^-17
±
7.86 10^-18
| 52.84
±
4.29
| 0.06
±
0.002
|
Constitutive: the induction point, in term of O.D.600, is under the minimum detectable value calculated by the aglorithm. This minimum value was estimated by running the algorithm on data acquired from a culture that constitutively produces GFP. For this reason, the devices labelled as constitutive can be considered as constitutive GFP producers.
*: in two of three experiments the self-induction failed, thus having a non-induced culture for all the cell densities. The standard errors were not computed for these cultures.
**: in one of three experiments the self-induction failed, thus having a non-induced culture for all the cell densities. The standard errors were computed computed on two independent experiments.
<partinfo>BBa_K300016</partinfo> is labelled with *, but probably induction failed in two of the three experiments because the culture didn't reach the ODstart point (the experiment was stopped before the culture reached the O.D.600 critical value).
Discussion: two self-inducible devices (<partinfo>BBa_K300010</partinfo> and <partinfo>BBa_K300009</partinfo>/<partinfo>BBa_F2620</partinfo>) were realized and characterized in many different experimental conditions:
- varying the strength of the promoter controlling the production of the signal molecule (Sender Modulation)
- varying the copy number of vectors bearing both Sender and Receiver circuits
- varying the growth medium (LB or M9)
A library of self-inducible promoters, able to start the production of the heterologous protein at a defined culture density, was realized. A graphical summary is reported in Figures:
 Average growth curve with ODstart evaluated by Threshold algorithm in LB |
 Average growth curve with ODstart evaluated by Threshold algorithm in M9 |
A model-based approach was proposed to estimate many interesting parameters, such as the HSL synthesis rate per cell and an algorithm was proposed, in order to evaluate the O.D.start for every self-inducible device.
In figure, the O.D.start and the HSL synthesis rate as a function of the strength of the promoter (RPU) controlling the signal molecule production are reported, both for <partinfo>BBa_K300010</partinfo> (Sender&Receiver device in HC plasmid) and for <partinfo>BBa_K300009</partinfo>/<partinfo>BBa_F2620</partinfo> (Sender device in LC plasmid in combination with Receiver device in HC plasmid).
 O.D.start and K_HSL as a function of RPUs of the promoters controlling the signal molecule production for <partinfo>BBa_K300010</partinfo> in high copy number plasmid in M9 medium |
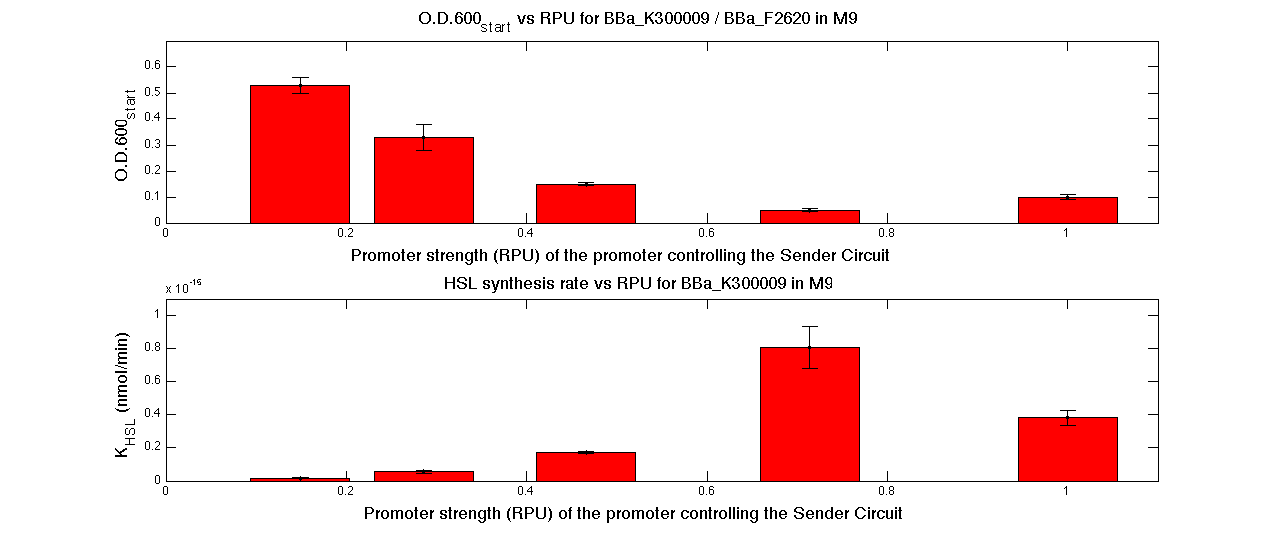 O.D.start and K_HSL as a function of RPUs of the promoters controlling the Signal Molucule production for <partinfo>BBa_K300010</partinfo> in low copy number plasmid used in combination with <partinfo>BBa_F2620</partinfo> in high copy plasmid in M9 medium |
A strong correlation between the promoter strength, previously measured in RPU, and the O.D.start is depicted by data. This is consistent with the expected behaviour of these parts, since the HSL synthesis rate results stronger at the increase of promoter's strength.
For RPU values greater or equal to 1, the expected behaviour is not confirmed anymore. This is probably due to the fact that too high synthesis rate of HSL are injurious for the cell.
A range of RPU values for promoters that control the Sender device was identified. When the PoPs-in signal belongs to this range, the expected behaviour is confirmed by experimental data.
The combination of promoter strength variation and plasmid copy number modulation allows the creation of a library of self-inducible devices, able to start the production of a target protein at different O.D._start values, in any cellular growth phase.
|
 "
"























