|
|
| Line 281: |
Line 281: |
| | |rowspan="8" width="250"|[[Image:Tp10_prediction.png|250px]]<br />3D structure of transportan<br /> [http://www.dbb.su.se/Faculty/Lena_M%C3%A4ler/Structural_basis_for_peptide-membrane_interactions www.dbb.su.se]--> | | |rowspan="8" width="250"|[[Image:Tp10_prediction.png|250px]]<br />3D structure of transportan<br /> [http://www.dbb.su.se/Faculty/Lena_M%C3%A4ler/Structural_basis_for_peptide-membrane_interactions www.dbb.su.se]--> |
| | ---- | | ---- |
| - |
| |
| - |
| |
| - | |}
| |
| - |
| |
| - | == Cell penetrating peptides ==
| |
| - |
| |
| - | This cell-penetrating peptides, (CPPs) may be used in N- and C-terminal fusions with full-length proteins to create transduction proteins with the ability to permeate the lipid bilayer of various cell types, making it a potential gene or protein delivery vector.
| |
| - |
| |
| - |
| |
| - | === TAT cell penetrating peptide (TAT) ===
| |
| - | Purified full-length TAT fusion proteins expressed in ''Escherichia coli'' have been shown to successfully translocate into several human cell types, including all cells found in whole blood, as well as bone marrow stem cells and osteoblasts, while still retaining the fused protein's activity ([http://www.ncbi.nlm.nih.gov/pubmed/9846587 Nagahara ''et al.'' 1998]). The mechanism for transduction over the bilipid membrane is still a matter of debate, but has been suggested to occur through macropinocytosis, a specialized form of endocytosis ([http://www.ncbi.nlm.nih.gov/pubmed/17913584 Gump and Dowdy, 2007]).
| |
| - | TAT is an 11-amino acid derivative from the Human Immunodeficiency Virus 1 (HIV-1) ''trans''-activating transcriptional activator (Tat) ([http://www.ncbi.nlm.nih.gov/pubmed/2849509 Green and Loewenstein, 1988]; [http://www.ncbi.nlm.nih.gov/pubmed/9846587 Nagahara ''et al.'' 1998]). This part was back translated from the corresponding amino acid sequence and optimized for expression in ''Escherichia coli''. Codon usage has been varied for repetitive amino acids to enable DNA synthesis.
| |
| - |
| |
| - | {|border="1" align="center" cellpadding="3" cellspacing="0"
| |
| - | !colspan="2"|Sequence
| |
| - | |-
| |
| - | |width="130"|'''Length'''
| |
| - | |width="130"|33 bp
| |
| - | |-
| |
| - | |'''GenBank'''
| |
| - | |<br />
| |
| - | |-
| |
| - | !colspan="2"|Peptide
| |
| - | |-
| |
| - | |'''Length'''
| |
| - | |11 aa
| |
| - | |-
| |
| - | |'''Size'''
| |
| - | |
| |
| - | |-
| |
| - | |colspan="2" align="center"|YGRKKRRQRRR
| |
| - | |-
| |
| - | |colspan="2"|Original protein first reported by [http://www.ncbi.nlm.nih.gov/pubmed/2849509 Green ''et al''. (1988)]<br />
| |
| - | and [http://www.ncbi.nlm.nih.gov/pubmed/2849510 Frankel ''et al''. (1988)] independently
| |
| - | |}
| |
| - |
| |
| - |
| |
| - | ----
| |
| - |
| |
| - | === Low molecular weight protamine (LMWP) ===
| |
| - | Enzymatically prepared LMWP chemically conjugated to ovalbumin (OVA) and bovine serum albumin (BSA) have previously been shown to penetrate the lipid bilayer of human keratinocytes, as well as to successfully permeate mouse skin epidermis ([http://www.ncbi.nlm.nih.gov/pubmed/20232417 Huang ''et al.'', 2010]). Furthermore, LMWP/pDNA complexes can efficiently penetrate into human embryonic kidney cells ([http://www.ncbi.nlm.nih.gov/pubmed/12898639 Park ''et al.'', 2003]). As LMWP has been shown to be neither toxic nor immunogenic ([http://www.ncbi.nlm.nih.gov/pubmed/11741268 Chang ''et al.'' a, 2001]; [http://www.ncbi.nlm.nih.gov/pubmed/11741269 Chang ''et al.'' b, 2001]; [http://www.ncbi.nlm.nih.gov/pubmed/11741270 Lee ''et al.'', 2001]), it may be used as a potential vaccine, drug or gene delivery vector.
| |
| - | LMWP is a 14-amino acid derivative from Rainbow trout (''Oncorhynchus mykiss'') protamine, an arginine-rich protein that replaces histones in chromatin during spermatogenesis ([http://www.ncbi.nlm.nih.gov/pubmed/3755398 McKay ''et al.'', 1986]; [http://www.ncbi.nlm.nih.gov/pubmed/10213181 Byun ''et al.'', 1999]). This part was back translated from the corresponding amino acid sequence and optimized for expression in ''Escherichia coli''. Codon usage has been varied for repetitive amino acids to enable DNA synthesis.
| |
| - |
| |
| - | {|border="1" align="center" cellpadding="3" cellspacing="0"
| |
| - | !colspan="2"|Sequence
| |
| - | |-
| |
| - | |width="130"|'''Length'''
| |
| - | |width="130"|42 bp
| |
| - | |-
| |
| - | |'''Patent Application'''
| |
| - | |[http://www.freepatentsonline.com/y2007/0071677.html 20070071677
| |
| - | ]<br />
| |
| - | |-
| |
| - | !colspan="2"|Peptide
| |
| - | |-
| |
| - | |'''Length'''
| |
| - | |14 aa
| |
| - | |-
| |
| - | |'''Size'''
| |
| - | |
| |
| - | |-
| |
| - | |colspan="2" align="center"|VSRRRRRRGGRRRR
| |
| - | |-
| |
| - | |colspan="2"|First reported by <unknown><br />
| |
| - | |}
| |
| - |
| |
| - |
| |
| - | ----
| |
| - |
| |
| - | === Transportan 10 (Tp10) ===
| |
| - | Chemically synthesized Tp10 peptides conjugated to different cargo, including pDNA and protein, have been shown to efficiently penetrate the lipid bilayer of both human and mouse cells ([http://www.ncbi.nlm.nih.gov/pubmed/15763630 Kilk ''et al.'', 2005]). Membrane permeation is both energy and temperature independent ([http://www.ncbi.nlm.nih.gov/pubmed/11718666 Hällbrink ''et al.'', 2001]). The exact mechanism for penetration is still unclear ([http://www.ncbi.nlm.nih.gov/pubmed/17218466 Yandek ''et al.'', 2007]).
| |
| - | Tp10 is a 21-amino acid derivative from the parent peptide transportan (originally known as galparan), which is a peptide chimera of the neuropeptide galanin and the wasp venom peptide mastoparan ([http://www.ncbi.nlm.nih.gov/pubmed/10930519 Soomets ''et al.'', 2000]; [http://www.ncbi.nlm.nih.gov/pubmed/8738882 Langel ''et al.'', 1996]). This part was back translated from the corresponding amino acid sequence and optimized for expression in ''Escherichia coli''. Codon usage has been varied for repetitive amino acids to enable DNA synthesis.
| |
| - |
| |
| - | {|border="1" align="center" cellpadding="3" cellspacing="0"
| |
| - | !colspan="2"|Sequence
| |
| - | |rowspan="10" width="250"|[[Image:Transportan.jpg|250px]]<br />3D structure of transportan<br /> [http://www.dbb.su.se/Faculty/Lena_M%C3%A4ler/Structural_basis_for_peptide-membrane_interactions www.dbb.su.se]
| |
| - | |-
| |
| - | |width="130"|'''Length'''
| |
| - | |width="130"|63 bp
| |
| - | |-
| |
| - | |'''Patent Application'''
| |
| - | |[http://www.freepatentsonline.com/y2008/0234183.html 20080234183]<br />
| |
| - | |-
| |
| - | !colspan="2"|Peptide
| |
| - | |-
| |
| - | |'''Length'''
| |
| - | |21 aa
| |
| - | |-
| |
| - | |'''Size'''
| |
| - | |
| |
| - | |-
| |
| - | |colspan="2" align="center"|AGYLLGKINLKALAALAKKIL
| |
| - | |-
| |
| - | |colspan="2"|First reported by <unknown><br />
| |
| - | |}
| |
| - |
| |
| - | {|border="1" align="right" cellpadding="3" cellspacing="0"
| |
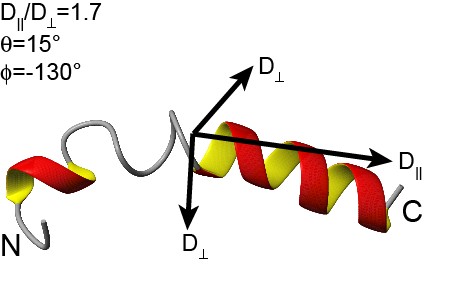
| - | |rowspan="10" width="250"|[[Image:Tp10_prediction.png|250px]]<br />3D structure of transportan<br /> [http://www.dbb.su.se/Faculty/Lena_M%C3%A4ler/Structural_basis_for_peptide-membrane_interactions www.dbb.su.se]
| |
| - | |}
| |
| - |
| |
| - |
| |
| - | ----
| |
| - |
| |
| - |
| |
| - | |}
| |
| - |
| |
| - | == Cell penetrating peptides ==
| |
| - |
| |
| - | This cell-penetrating peptides, (CPPs) may be used in N- and C-terminal fusions with full-length proteins to create transduction proteins with the ability to permeate the lipid bilayer of various cell types, making it a potential gene or protein delivery vector.
| |
| - |
| |
| - |
| |
| - | === TAT cell penetrating peptide (TAT) ===
| |
| - | Purified full-length TAT fusion proteins expressed in ''Escherichia coli'' have been shown to successfully translocate into several human cell types, including all cells found in whole blood, as well as bone marrow stem cells and osteoblasts, while still retaining the fused protein's activity ([http://www.ncbi.nlm.nih.gov/pubmed/9846587 Nagahara ''et al.'' 1998]). The mechanism for transduction over the bilipid membrane is still a matter of debate, but has been suggested to occur through macropinocytosis, a specialized form of endocytosis ([http://www.ncbi.nlm.nih.gov/pubmed/17913584 Gump and Dowdy, 2007]).
| |
| - | TAT is an 11-amino acid derivative from the Human Immunodeficiency Virus 1 (HIV-1) ''trans''-activating transcriptional activator (Tat) ([http://www.ncbi.nlm.nih.gov/pubmed/2849509 Green and Loewenstein, 1988]; [http://www.ncbi.nlm.nih.gov/pubmed/9846587 Nagahara ''et al.'' 1998]). This part was back translated from the corresponding amino acid sequence and optimized for expression in ''Escherichia coli''. Codon usage has been varied for repetitive amino acids to enable DNA synthesis.
| |
| - |
| |
| - | {|border="1" align="center" cellpadding="3" cellspacing="0"
| |
| - | !colspan="2"|Sequence
| |
| - | |-
| |
| - | |width="130"|'''Length'''
| |
| - | |width="130"|33 bp
| |
| - | |-
| |
| - | |'''GenBank'''
| |
| - | |<br />
| |
| - | |-
| |
| - | !colspan="2"|Peptide
| |
| - | |-
| |
| - | |'''Length'''
| |
| - | |11 aa
| |
| - | |-
| |
| - | |'''Size'''
| |
| - | |
| |
| - | |-
| |
| - | |colspan="2" align="center"|YGRKKRRQRRR
| |
| - | |-
| |
| - | |colspan="2"|First reported by <unknown><br />
| |
| - | |}
| |
| - |
| |
| - |
| |
| - | ----
| |
| - |
| |
| - | === Low molecular weight protamine (LMWP) ===
| |
| - | Enzymatically prepared LMWP chemically conjugated to ovalbumin (OVA) and bovine serum albumin (BSA) have previously been shown to penetrate the lipid bilayer of human keratinocytes, as well as to successfully permeate mouse skin epidermis ([http://www.ncbi.nlm.nih.gov/pubmed/20232417 Huang ''et al.'', 2010]). Furthermore, LMWP/pDNA complexes can efficiently penetrate into human embryonic kidney cells ([http://www.ncbi.nlm.nih.gov/pubmed/12898639 Park ''et al.'', 2003]). As LMWP has been shown to be neither toxic nor immunogenic ([http://www.ncbi.nlm.nih.gov/pubmed/11741268 Chang ''et al.'' a, 2001]; [http://www.ncbi.nlm.nih.gov/pubmed/11741269 Chang ''et al.'' b, 2001]; [http://www.ncbi.nlm.nih.gov/pubmed/11741270 Lee ''et al.'', 2001]), it may be used as a potential vaccine, drug or gene delivery vector.
| |
| - | LMWP is a 14-amino acid derivative from Rainbow trout (''Oncorhynchus mykiss'') protamine, an arginine-rich protein that replaces histones in chromatin during spermatogenesis ([http://www.ncbi.nlm.nih.gov/pubmed/3755398 McKay ''et al.'', 1986]; [http://www.ncbi.nlm.nih.gov/pubmed/10213181 Byun ''et al.'', 1999]). This part was back translated from the corresponding amino acid sequence and optimized for expression in ''Escherichia coli''. Codon usage has been varied for repetitive amino acids to enable DNA synthesis.
| |
| - |
| |
| - | {|border="1" align="center" cellpadding="3" cellspacing="0"
| |
| - | !colspan="2"|Sequence
| |
| - | |-
| |
| - | |width="130"|'''Length'''
| |
| - | |width="130"|42 bp
| |
| - | |-
| |
| - | |'''GenBank'''
| |
| - | |<br />
| |
| - | |-
| |
| - | !colspan="2"|Peptide
| |
| - | |-
| |
| - | |'''Length'''
| |
| - | |14 aa
| |
| - | |-
| |
| - | |'''Size'''
| |
| - | |
| |
| - | |-
| |
| - | |colspan="2" align="center"|VSRRRRRRGGRRRR
| |
| - | |-
| |
| - | |colspan="2"|First reported by <unknown><br />
| |
| - | |}
| |
| - |
| |
| - |
| |
| - | ----
| |
| - |
| |
| - | === Transportan 10 (Tp10) ===
| |
| - | Chemically synthesized Tp10 peptides conjugated to different cargo, including pDNA and protein, have been shown to efficiently penetrate the lipid bilayer of both human and mouse cells ([http://www.ncbi.nlm.nih.gov/pubmed/15763630 Kilk ''et al.'', 2005]). Membrane permeation is both energy and temperature independent ([http://www.ncbi.nlm.nih.gov/pubmed/11718666 Hällbrink ''et al.'', 2001]). The exact mechanism for penetration is still unclear ([http://www.ncbi.nlm.nih.gov/pubmed/17218466 Yandek ''et al.'', 2007]).
| |
| - | Tp10 is a 21-amino acid derivative from the parent peptide transportan (originally known as galparan), which is a peptide chimera of the neuropeptide galanin and the wasp venom peptide mastoparan ([http://www.ncbi.nlm.nih.gov/pubmed/10930519 Soomets ''et al.'', 2000]; [http://www.ncbi.nlm.nih.gov/pubmed/8738882 Langel ''et al.'', 1996]). This part was back translated from the corresponding amino acid sequence and optimized for expression in ''Escherichia coli''. Codon usage has been varied for repetitive amino acids to enable DNA synthesis.
| |
| - |
| |
| - | {|border="1" align="center" cellpadding="3" cellspacing="0"
| |
| - | !colspan="2"|Sequence
| |
| - | |rowspan="10" width="250"|[[Image:Transportan.jpg|250px]]<br />3D structure of transportan<br /> [http://www.dbb.su.se/Faculty/Lena_M%C3%A4ler/Structural_basis_for_peptide-membrane_interactions www.dbb.su.se]
| |
| - | |-
| |
| - | |width="130"|'''Length'''
| |
| - | |width="130"|63 bp
| |
| - | |-
| |
| - | |'''GenBank'''
| |
| - | |<br />
| |
| - | |-
| |
| - | !colspan="2"|Peptide
| |
| - | |-
| |
| - | |'''Length'''
| |
| - | |21 aa
| |
| - | |-
| |
| - | |'''Size'''
| |
| - | |
| |
| - | |-
| |
| - | |colspan="2" align="center"|AGYLLGKINLKALAALAKKIL
| |
| - | |-
| |
| - | |colspan="2"|First reported by <unknown><br />
| |
| - | |}
| |
| - |
| |
| - |
| |
| - | ----
| |
| - |
| |
| | | | |
| | |} | | |} |
| | | | |
| | {{Stockholm/Footer}} | | {{Stockholm/Footer}} |








 "
"