Team:RMIT Australia/Project
From 2010.igem.org
(→The Project) |
(→Creation of the Taq Polymerase Part) |
||
| Line 451: | Line 451: | ||
<br> | <br> | ||
| - | TAQ was chosen as a carrier molecule because of it's thermostability at high temperatures | + | TAQ polymerase was chosen as a carrier molecule because of it's physico-chemical properties - most notably, TAQ's thermostability at high temperatures; being able to fold and unfold repeatedly and through temperature gradients up to 95degC. TAQ polymerase is a DNA-dependent DNA polymerase, using any DNA strand as a substrate and, conceivably, TAQ expression levels within E.coli will be limited by toxic effects the protein will have when binding to genomic DNA and disrupting cellular functions. Fortunately, the natural polymerase function is not required for this project and so mutagenesis was chosen to knock-out DNA-binding, but retaining the favourable physico-chemical properties of TAQ. |
| + | |||
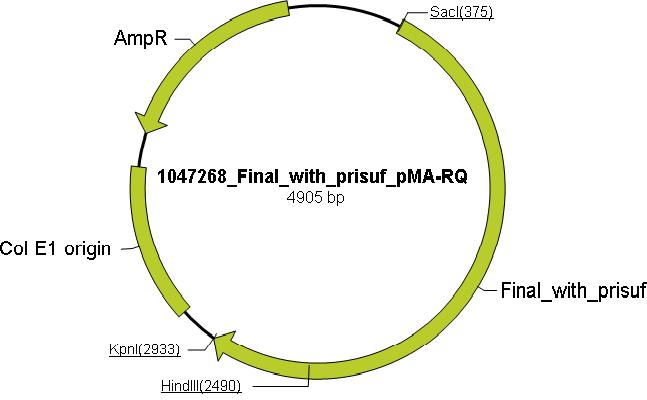
| + | Using the ExPASy proteomics server the aliphatic index, instability index, extinction coefficient and theoretical isoelectric point were determined to distinguish mutations that increase the thermostability and through literature research, amino acids which are essential for catalytic activity of TAQ were identified (residue No. 659 arginine to isoleucine and residue No. 663 lysine to leucine). Mutating these residues would render TAQ incapable of polymerase activity but retain thermostability. Mutations to enable ligation independent cloning (LIC) were added to the N and the C terminus (see LIC section for more information), along with the prefix(P) and the suffix(S). These mutation were incorporated into TAQ sequence to produce the mutTAQ sequence (see modeling for more information). The sequence was then sent to Mr. Gene (Gene Art) for the production of the (pMA-RQ) vector containing the muTAQ sequence conforming to standard 10 cloning (below is a pictorial representation of the vector containing mutTAQ). | ||
| + | |||
Revision as of 09:28, 27 October 2010


The Project
The RMIT University iGEM project will attempt to create a peptide expression platform using Synthetic Biology. The recombinant production of protein is routine in science and industry. Despite protein expression being achievable, peptide expression is frequently too complicated or not economically viable; peptides are small and often contain very little – or even no secondary structure – making them highly susceptible to proteolysis and other cellular processes.
In the laboratory for research purposes, carrier proteins with affinity for a purification matrix are commonly employed, allowing “protection” of the peptide intracellularly and providing a defined bioprocess. There are several limitations to this technology that curtails the scale: Firstly, the process is extremely inefficient as it expends the feedstock and cellular metabolism to essentially produce large amounts of a worthless carrier protein (as much as 98% of the protein-peptide fused product by mass)., Secondly, affinity resins used to capture the carrier protein are expensive and rarely congenial to scalable chromatography unit operations such as expanded bed chromatography., Thirdly, there is a requirement to separate the peptide from the carrier protein that often utilises a protease. Proteases are expensive at elevated scales, and despite popular beliefs are actually inefficient with best estimates ranging in ratio yields of 1:40 – 1:200 for the desired product as a “good” outcome due to inefficiencies, non-specific cleavage or loss during purification. For these reasons there are very few commodity peptides made recombinantly or even by semi-synthetic methods, with solid-phase peptide synthesis remaining the industry benchmark.
The RMIT University iGEM project poses the question: Can the bottlenecks of purification and proteolysis be removed from the workflow to economically produce a peptide and commodity carrier protein? In this project we propose the thermostable protein Taq polymerase may be used as a carrier molecule to allow for the production of a peptide that will be attached to the Taq commodity enzyme. The peptide-Taq fusion will be attached by a thermolabile bond – an Asp/Pro bond in between the peptide and the Taq Polymerase, with the intention of releasing the peptide-Taq fusion products by thermolysis. The hypothetical bioprocess will involve heating the bacteria below the threshold of the thermolabile bond, then following clarification or polishing, heating above the threshold to separate the peptide from the Taq protein. The peptide and Taq may then be purified allowing two income streams from the manufacture.
Our project seeks to produce several unique parts for the registry: Firstly, a plasmid and system for producing Taq polymerase as a useful part for future iGEM projects undertaking PCR as part of their experiments., Secondly, an expression device will be provided to the registry that will allow peptides to be inserted easily and quickly by a process of ligand independent cloning into the device to produce any peptide at will, or even allow the rapid and economical production of a library of peptides for drug design and screening.
Project Details
Ligation Independent Cloning
Ligation independent cloning (LIC) is a new procedure that exploits the 3’-5’ exonuclease activity of DNA polymerases to produce sequence-specific overhangs for target primer insertion. In the LIC procedure, the DNA is cut with a restriction enzyme(s) and DNA polymerase is added in the presence of only one dNTP. In the absence of all other dNTPs, the equilibrium of the polymerase activity shift to exonuclease activity and nucleotides are removed from the point of the restriction digest until the polymerase encounters a nucleotide that corresponds to the single dNTP present. At this point the exonulease activity stops and is counteracted by the 5’-3’ polymerisation activity which prevents further changes to the DNA due to only one dNTP being present. This results in a polymerase-digested vector with non-homologous sequences of sticky-ends. Primers can then be designed with complementarity for the sticky-ends, allowing for the insertion of a desired peptide sequence within the vector by simply annealing the primers to the vector and transformation into cells.
The thermostable protein Taq polymerase was chosen as a carrier molecule, to facilitate the production of a peptide as a peptide-Taq fusion protein separated by a thermolabile bond, such as an Asp/Pro bond. The proposed bioprocess would then involve heating the bacteria below the threshold of the thermolabile bond for simplified lysis, then following clarification or polishing, heating above the threshold of the thermolabile bond to separate the peptide from the Taq protein allowing use expression immediately in assays, or providing a simple starting source for final purification.
The peptide plasmid was also designed with the intention of allowing peptides to be inserted easily and quickly by a process of Ligation Independent Cloning (LIC) (refns) to produce a desired peptide at will, or even allow the rapid and economical production of a library of peptides for drug design and screening. Two test peptides were chosen to explore the capacity of the peptide expression platform being Losartan and arginine vasopressin. Arginine vasopressin and the angiotensin antagonist Losartan were chosen because they both had short amino acid sequences that we could design primers for.
With Taq, it is feasible that the first and last 10 amino acids of Taq may be deleted without greatly influencing the 3D structure . The first 10 amino acids are not present in the original crystal structure and both the N- and C- termini are in regions which project a putative peptide more generally away from the protein structure, with relatively few intramolecular native contacts observable in this region. To further facilitate the separation and projection of expressed peptides away from the native protein structure, a hydrophilic spacer arm was also included in the design of mutTaq at the N- and C- termini consisting of a simple GSSG motif that produces a known extended conformation of ~9.88Å .
Because Taq has a DNA sequence with an unusually rich GC content it was determine that the appropriate dNTP to generate the specific overhangs should be either dATP or dTTP as this allows a lengthy digestion by a DNA polymerase of >19 nucleotides. The generation of these sticky ends would require digestion by the polymerase into the coding region for both the N- or C- terminus of the mutTaq but also into important expression features of the vector such as the stop codon, start codon and ribosomal binding site. An NdeI restriction site (CA^TATG) was therefore designed at the start codon of mutTaq (5’ coding region) and nucleotides for a GSSG spacer arm and AvrII restriction site (C^CTAGG) were designed at the 3’ coding region. The NdeI and AvrII restriction enzymes were considered favourable restriction enzymes to employ for process control and flexibility as they can be heat inactivated at 65ºC and 80ºC respectively, have more broad buffer compatibilities, are not sensitive to methylation sensitivities (dam, dcm, CpG) and are suitable for extended digestion (e.g. >8h or overnight).
Peptides
The peptides chosen for this study were Losartan and arginine vasopressin.
Losartan elicits effects in the renin-angiotensin system; in this system angiotensin is formed by the action of renin on angiotensinogen and the conversion between Angiotensin I and angiotensin II is regulated by dipeptidyl carboxypeptidase angiotensin-converting enzyme (ACE). This renin-angiotensin system regulates electrolyte balance and arterial blood pressure by the effects of vasoconstriction and the release of aldosterone from the adrenal cortex. Losartan is considered the drug of choice in hypertension control and for the protection of kidneys in diabetes mellitus by acting on the renin–angiotensin system through antagonism of angiotensin II AT1 receptors resulting in vasodilation.
The second peptide produced was arginine vasopressin which is an antidiuretic hormone secreted from the posterior pituitary gland that exerts two important biological functions; (i) potent vasoconstriction and (ii) as the main regulator of water homeostasis and extracellular fluid volume. The later antidiuretic properties of arginine vasopressin are the most biologically important, exerting therapeutic effects in the kidney and the vascular system and therefore being widely used in the treatment of diabetes insipidus. G- coupled protein receptor (GPCR) agonists such as vasopressin, account for 40% of prescription pharmaceuticals on the market and are the core of medicine as GPCRs are the most investigated target sites in the pharmaceutical industry. For these reasons and for its simplicity to assay, it has been chosen as a candidate to test the efficacy of the new technology. Calcium ion assays are used to detect the activation of GCPRs as they are naturally produced upon activation of GPCRs coupled to Gq, the G protein involved in the activation of the arginine vasopressin receptor 1 that causes vasoconstriction. Increased intracellular calcium levels infer that the peptide has been produced and expressed. Screening for cyclic adeno monophosphate (cAMP), a second messenger produced when the receptor is activated, indicates the presence of a peptide such as vasopressin that has triggered this activation with the coupling of G proteins. Luminescent tags bind to the cAMP to allow for its detection.
Creation of the Taq Polymerase Part
TAQ polymerase was chosen as a carrier molecule because of it's physico-chemical properties - most notably, TAQ's thermostability at high temperatures; being able to fold and unfold repeatedly and through temperature gradients up to 95degC. TAQ polymerase is a DNA-dependent DNA polymerase, using any DNA strand as a substrate and, conceivably, TAQ expression levels within E.coli will be limited by toxic effects the protein will have when binding to genomic DNA and disrupting cellular functions. Fortunately, the natural polymerase function is not required for this project and so mutagenesis was chosen to knock-out DNA-binding, but retaining the favourable physico-chemical properties of TAQ.
Using the ExPASy proteomics server the aliphatic index, instability index, extinction coefficient and theoretical isoelectric point were determined to distinguish mutations that increase the thermostability and through literature research, amino acids which are essential for catalytic activity of TAQ were identified (residue No. 659 arginine to isoleucine and residue No. 663 lysine to leucine). Mutating these residues would render TAQ incapable of polymerase activity but retain thermostability. Mutations to enable ligation independent cloning (LIC) were added to the N and the C terminus (see LIC section for more information), along with the prefix(P) and the suffix(S). These mutation were incorporated into TAQ sequence to produce the mutTAQ sequence (see modeling for more information). The sequence was then sent to Mr. Gene (Gene Art) for the production of the (pMA-RQ) vector containing the muTAQ sequence conforming to standard 10 cloning (below is a pictorial representation of the vector containing mutTAQ).
Making a Controlled T7 Expression Vector
We needed a strong expression vector with the functionality of regulatory sites.
o To create this we started with part BBa_J04500.
o It contains CAP and Lac binding site, both being able to be regulated. Allowing for more control over degree of expression.
o Between the -35 and -10 sites of the promoter region, T7 was the ideal promoter to be inserted. This would provide the expression vector with the strength we required while preventing RNA pol from binding.
1.Firstly the -35 site was mutated to an Afl II restriction site, after testing with digestion and electrophoresis.
2.We continued with the second mutagenesis. This was at the site of -10 within the promoter and it was mutated to a ClaI restriction site.
3.At this point, ideally both the sites were restriction sites so we ran a double digest and ligated the T7 promoter, inserting it between the -35 and -10 sites.
== Results == "
"