Team:ETHZ Basel/Modeling/Chemotaxis
From 2010.igem.org
(→Modeling of the chemotaxis pathway) |
|||
| Line 7: | Line 7: | ||
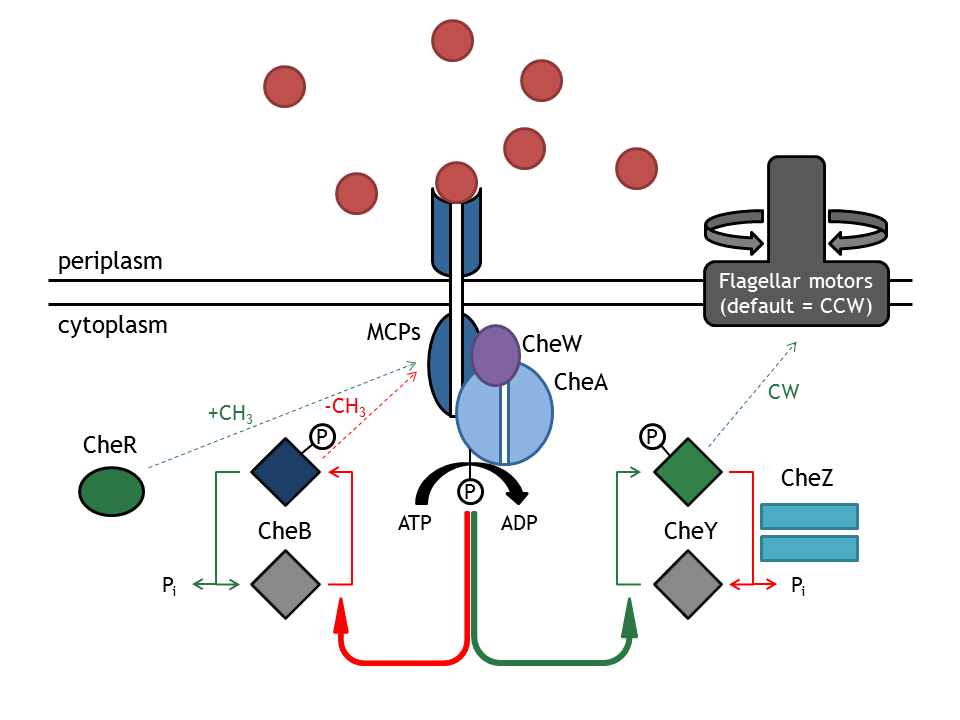
[[Image:ETHZ_Basel_chemotactical_network.png|thumb|400px|'''Schematical overview of the chemotaxis pathway.''' MCPs refers to the membrane receptor proteins and Che to the intracellular chemotactical proteins.]] | [[Image:ETHZ_Basel_chemotactical_network.png|thumb|400px|'''Schematical overview of the chemotaxis pathway.''' MCPs refers to the membrane receptor proteins and Che to the intracellular chemotactical proteins.]] | ||
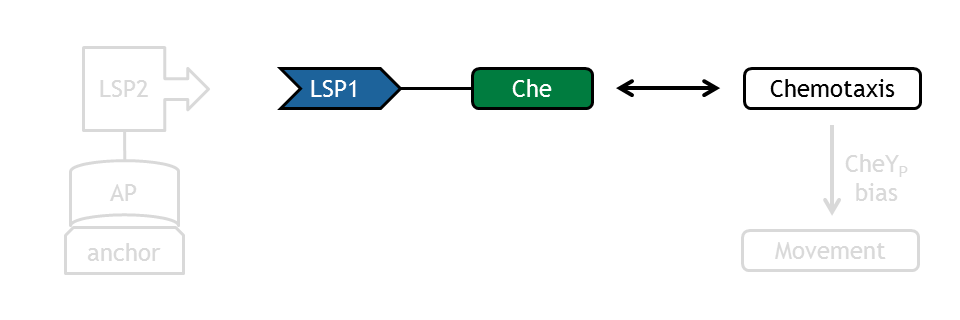
| - | The [[Team:ETHZ_Basel/Biology/Molecular_Mechanism|chemotaxis receptor pathway]] in ''E. coli'' is quite complex. Published models of chemotaxis [[Team:ETHZ_Basel/Modeling/Chemotaxis#References|[1]]], [[Team:ETHZ_Basel/Modeling/Chemotaxis#References|[2]]], [[Team:ETHZ_Basel/Modeling/Chemotaxis#References|[3]]], [[Team:ETHZ_Basel/Modeling/Chemotaxis#References|[4]]] thus have to use many assumptions in order to answer the investigated question: How does the output species (CheYp bias) react to perturbations of upstream species? It was therefore decided to | + | The [[Team:ETHZ_Basel/Biology/Molecular_Mechanism|chemotaxis receptor pathway]] in ''E. coli'' is quite complex. Published models of chemotaxis [[Team:ETHZ_Basel/Modeling/Chemotaxis#References|[1]]], [[Team:ETHZ_Basel/Modeling/Chemotaxis#References|[2]]], [[Team:ETHZ_Basel/Modeling/Chemotaxis#References|[3]]], [[Team:ETHZ_Basel/Modeling/Chemotaxis#References|[4]]] thus have to use many assumptions in order to answer the investigated question: |
| + | |||
| + | * How does the output species (CheYp bias) react to perturbations of upstream species? | ||
| + | |||
| + | It was therefore decided to adapt and extend four different models based on published approaches [[Team:ETHZ_Basel/Modeling/Chemotaxis#References|[1]]], [[Team:ETHZ_Basel/Modeling/Chemotaxis#References|[2]]], [[Team:ETHZ_Basel/Modeling/Chemotaxis#References|[3]]], [[Team:ETHZ_Basel/Modeling/Chemotaxis#References|[4]]] to be able to achieve a more general consensus prediction of the chemotaxis behavior in E. lemming. | ||
The chemotaxis network represents the main decision factor in bacterial movement and therefore, it received special attention for the [[Team:ETHZ_Basel/Modeling/Experimental_Design|experimental design]] of E. lemming. | The chemotaxis network represents the main decision factor in bacterial movement and therefore, it received special attention for the [[Team:ETHZ_Basel/Modeling/Experimental_Design|experimental design]] of E. lemming. | ||
| Line 14: | Line 18: | ||
== Spiro et al. (1997) [[Team:ETHZ Basel/Modeling/Chemotaxis#References|[1]]] == | == Spiro et al. (1997) [[Team:ETHZ Basel/Modeling/Chemotaxis#References|[1]]] == | ||
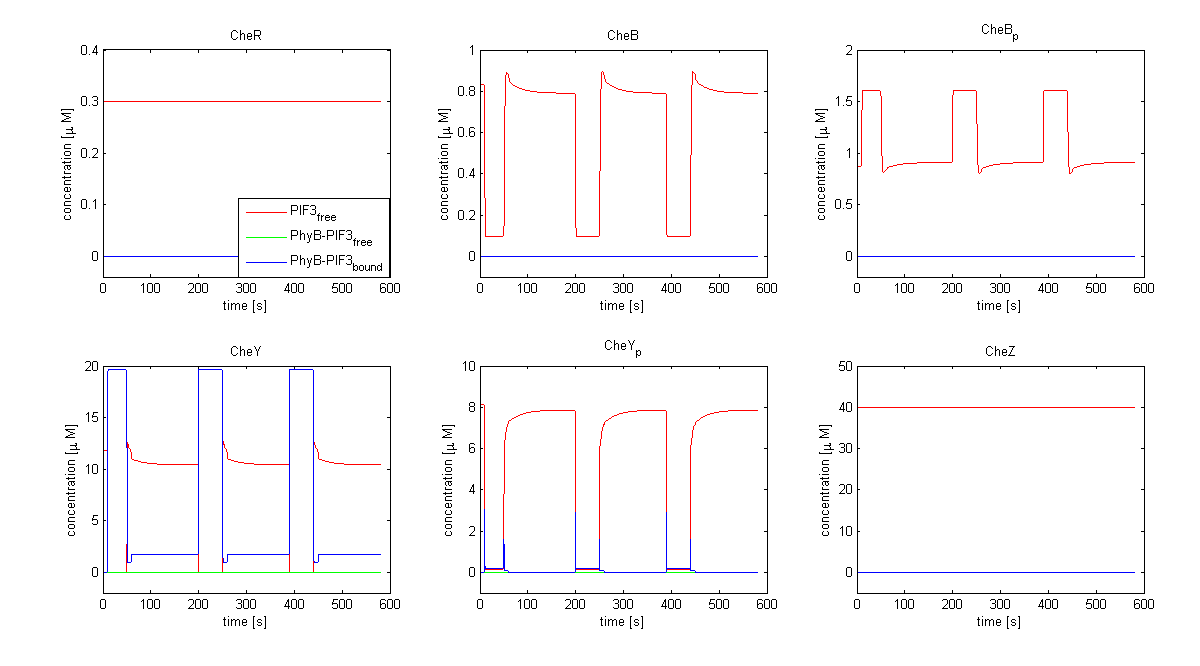
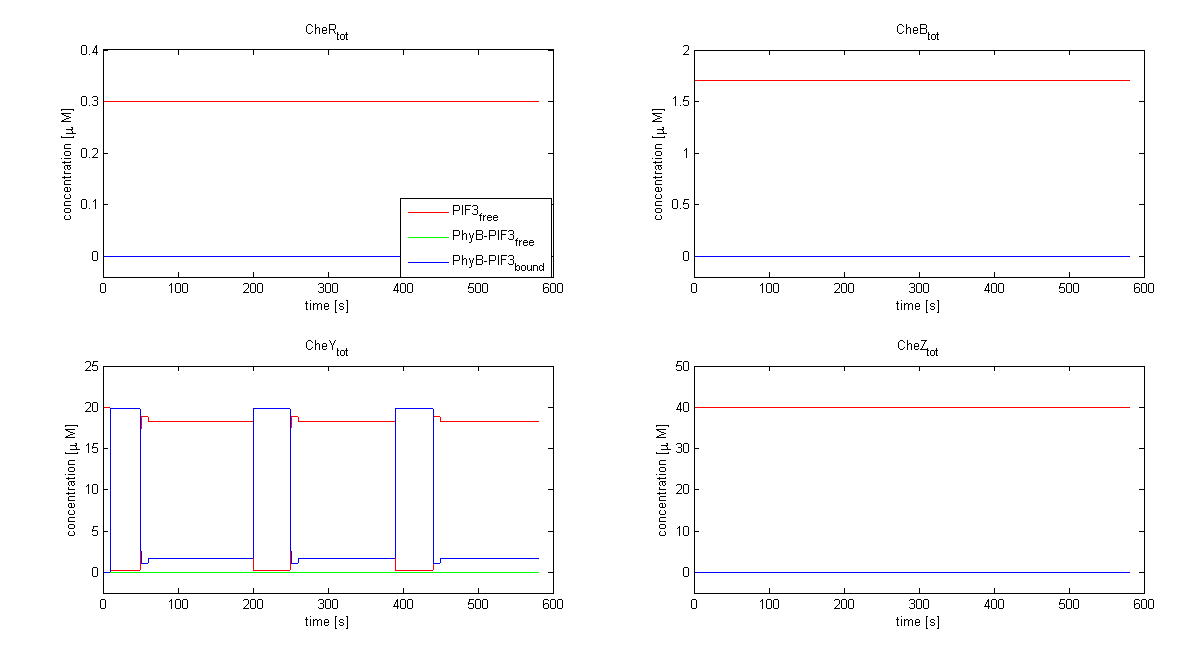
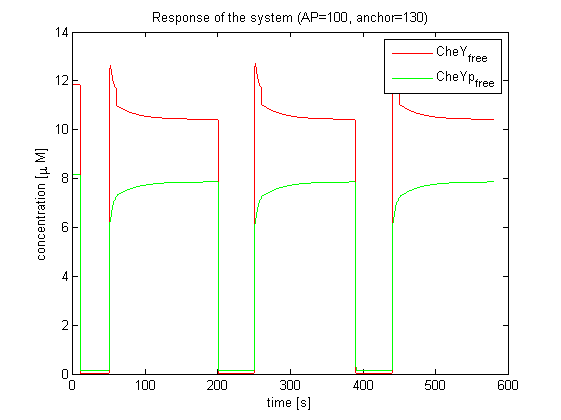
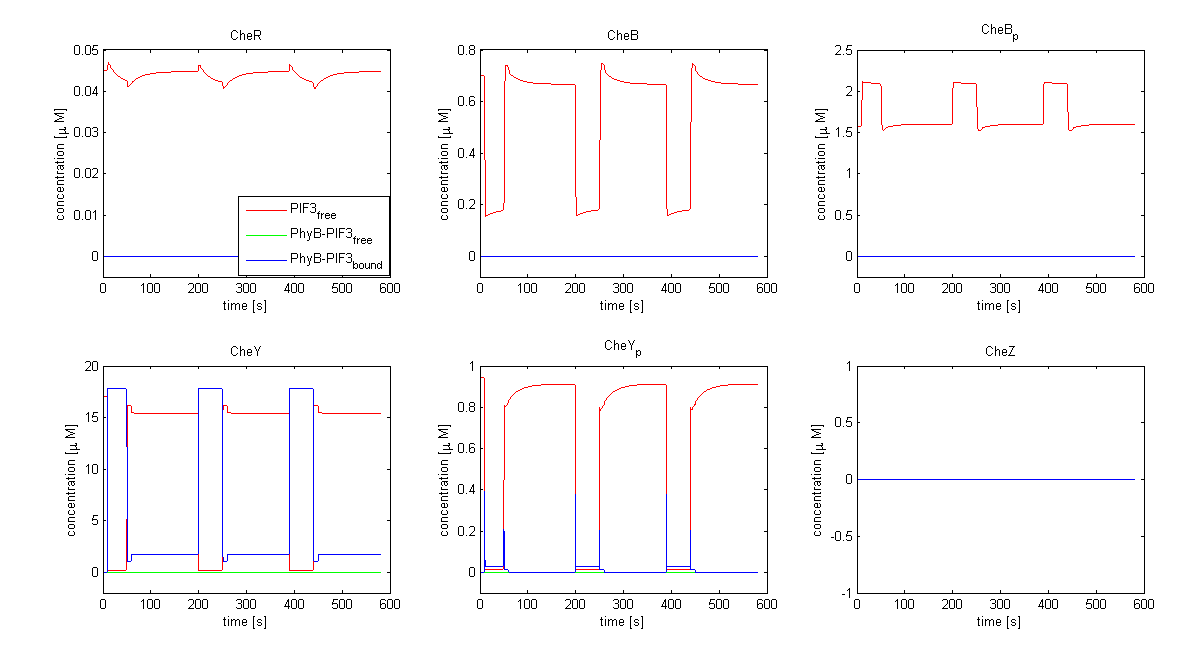
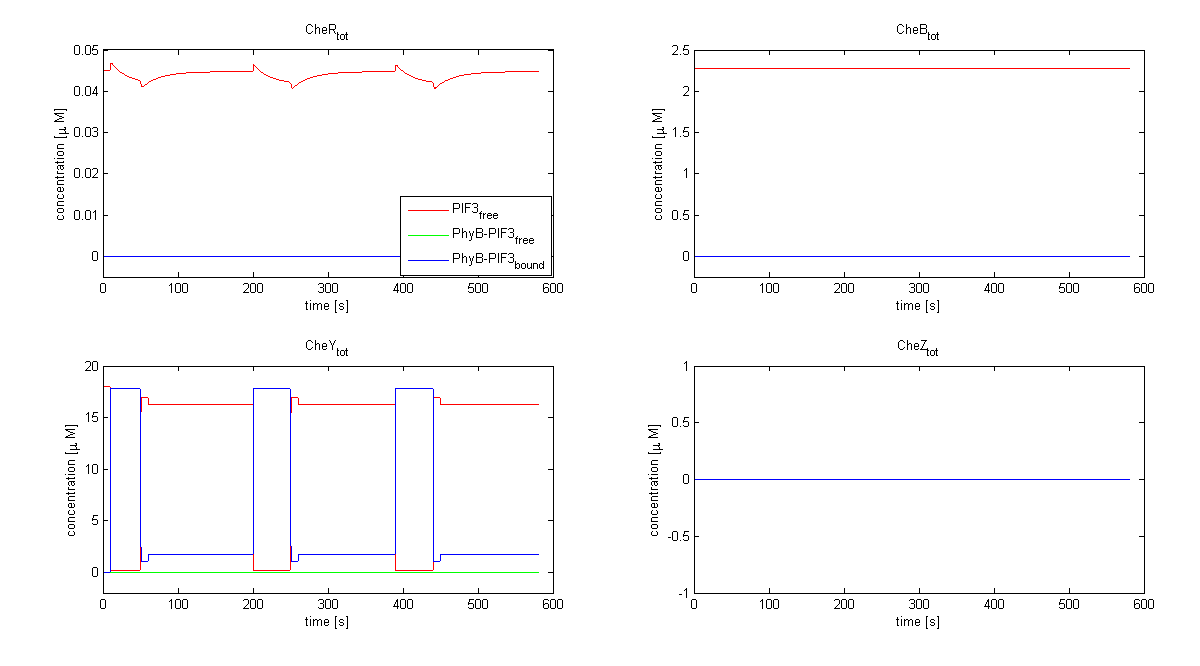
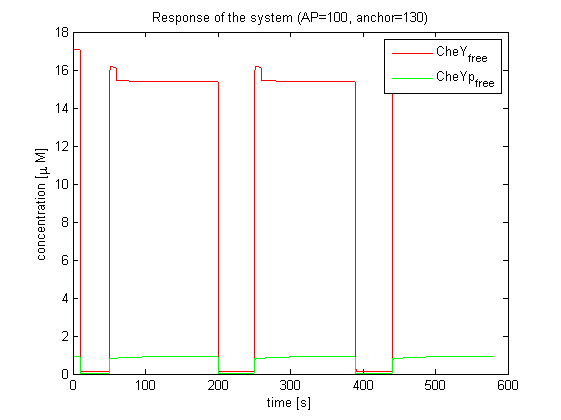
| - | The model of Spiro et al. (1997) [[Team:ETHZ Basel/Modeling/Chemotaxis#References|[1]]] has been adapted to identify candidates of the chemotaxis receptor pathway by enabling removal of a species upon light | + | The model of Spiro et al. (1997) [[Team:ETHZ Basel/Modeling/Chemotaxis#References|[1]]] has been adapted to identify candidates of the chemotaxis receptor pathway by enabling removal of a species upon light induction. It basically predicts that all Che proteins (CheR, B, Y and Z) would be a suitable candidate. Threshold is the value of the species concentration which is reached after light pulse induction. For CheR and CheY, the concentration of CheYp drops more than Delta (initial value - threshold), for CheB and CheZ it increases more than Delta. For all Che proteins, the concentrations stay below/above the threshold, until they are deactivated with far-red light. The best results were obtained for assuming a high ligand concentration (saturation, the methylation level of the receptors is high). For CheY and CheZ the reaction times were much faster than for CheB and CheR. |
[[Image:ETHZ_Basel_chemotaxis_spiro_1.png|thumb|center|833px|'''Che protein species predicted by the model of Spiro et al. (1997)''' CheY is coupled to PIF3. PhyB is present in a concentration of 100 uM, anchor in a concentration of 130 uM. Medium asparate levels (10^-6 uM) were chosen.]] | [[Image:ETHZ_Basel_chemotaxis_spiro_1.png|thumb|center|833px|'''Che protein species predicted by the model of Spiro et al. (1997)''' CheY is coupled to PIF3. PhyB is present in a concentration of 100 uM, anchor in a concentration of 130 uM. Medium asparate levels (10^-6 uM) were chosen.]] | ||
Revision as of 10:24, 26 October 2010
Modeling of the chemotaxis pathway
The chemotaxis receptor pathway in E. coli is quite complex. Published models of chemotaxis [1], [2], [3], [4] thus have to use many assumptions in order to answer the investigated question:
- How does the output species (CheYp bias) react to perturbations of upstream species?
It was therefore decided to adapt and extend four different models based on published approaches [1], [2], [3], [4] to be able to achieve a more general consensus prediction of the chemotaxis behavior in E. lemming.
The chemotaxis network represents the main decision factor in bacterial movement and therefore, it received special attention for the experimental design of E. lemming.
For the combined model implementation, only two chemotaxis models have been further investigated to focus on implementation and extension of the original models: Spiro et al. (1997) and Mello & Tu (2003).
Spiro et al. (1997) [1]
The model of Spiro et al. (1997) [1] has been adapted to identify candidates of the chemotaxis receptor pathway by enabling removal of a species upon light induction. It basically predicts that all Che proteins (CheR, B, Y and Z) would be a suitable candidate. Threshold is the value of the species concentration which is reached after light pulse induction. For CheR and CheY, the concentration of CheYp drops more than Delta (initial value - threshold), for CheB and CheZ it increases more than Delta. For all Che proteins, the concentrations stay below/above the threshold, until they are deactivated with far-red light. The best results were obtained for assuming a high ligand concentration (saturation, the methylation level of the receptors is high). For CheY and CheZ the reaction times were much faster than for CheB and CheR.
Mello & Tu (2003) [2]
The model of Mello & Tu (2003) differs from Spiro et al. (1997) in a way, that it is able to reach perfect and near-perfect adaptation. They investigated robustness of this system, which covers the effect of attractant binding through the phosphorylation of CheY. Governing ODEs are derived by applying the law of mass action to the known reactions. Five states of methylation and demethylation of the attractant-bound and free receptors are considered. The Mello & Tu (2003) model has been adapted to support our approach and shows similar behavior compared to the adapted Spiro et al. (1997) model.
References
[1] [http://www.pnas.org/content/94/14/7263.full Spiro et al: A model of excitation and adaptation in bacterial chemotaxis. PNAS 1997 94;14;7263-7268.]
[2] [http://www.cell.com/biophysj/retrieve/pii/S0006349503700216 Mello & Tu: Perfect and Near-Perfect Adaptation in a Model of Bacterial Chemotaxis. Biophysical Journal 2003 84;5;2943-2956.]
[3] [http://www.plosbiology.org/article/info:doi/10.1371/journal.pbio.0020049 Rao et al: Design and Diversity in Bacterial Chemotaxis. PLoS Biol 2004;2;2;239-252.]
[4] [http://www.nature.com/nature/journal/v387/n6636/abs/387913a0.html Barkai & Leibler: Robustness in simple biochemical networks. Nature 1997;387;913-917.]
 "
"