Team:UNIPV-Pavia/Material Methods/Measurements/Tecan/test28settembre
From 2010.igem.org
m (→RESULTS) |
m (→RESULTS) |
||
| Line 51: | Line 51: | ||
</tr> | </tr> | ||
<tr align="center"> | <tr align="center"> | ||
| - | <td>I63<br/> | + | <td>I63<br/> not induced</td><td>74</td> |
</tr> | </tr> | ||
<tr align="center"> | <tr align="center"> | ||
| Line 57: | Line 57: | ||
</tr> | </tr> | ||
<tr align="center"> | <tr align="center"> | ||
| - | <td>I64<br/> | + | <td>I64<br/>not induced</td><td>72</td> |
</tr> | </tr> | ||
<tr align="center"> | <tr align="center"> | ||
| Line 71: | Line 71: | ||
[[Image:UNIPV10 SScellM28settembre.png|thumb|500px |center|Mean of (dGFP/dt)/O.D.600 (under the hypothesis that half-life of GFP is longer than experiment observation)]] | [[Image:UNIPV10 SScellM28settembre.png|thumb|500px |center|Mean of (dGFP/dt)/O.D.600 (under the hypothesis that half-life of GFP is longer than experiment observation)]] | ||
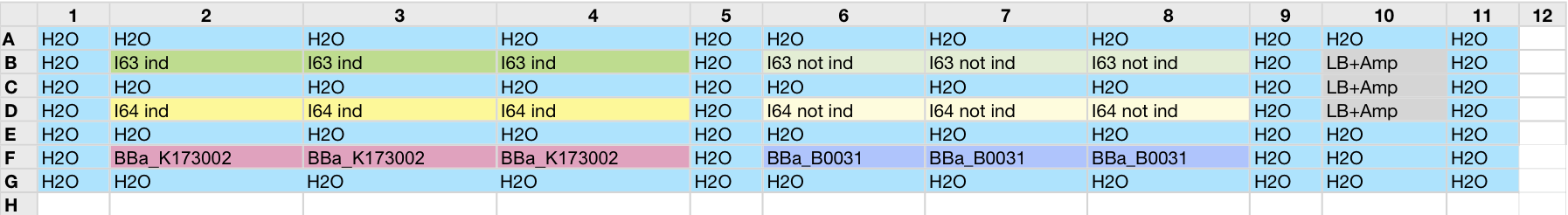
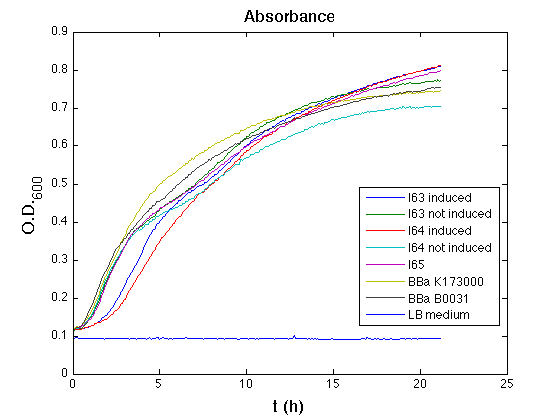
| - | All cell cultures showed a similar growth curve and doubling time was computed as described [[Team:UNIPV-Pavia/Parts/Characterization#Doubling_time_evaluation|here]] in order to have informations about the burden due to synthesis of such fusion proteins. It's possible to see that all doubling time are very similar except for induced cultures. In this case doubling time is much higher than posite control and | + | All cell cultures showed a similar growth curve and doubling time was computed as described [[Team:UNIPV-Pavia/Parts/Characterization#Doubling_time_evaluation|here]] in order to have informations about the burden due to synthesis of such fusion proteins. It's possible to see that all doubling time are very similar except for induced cultures. In this case doubling time is much higher than posite control and not induced cultures; so it's possible to assert that in this case there's a kind of metabolic burden higher than in the others, maybe because of the inducible system. |
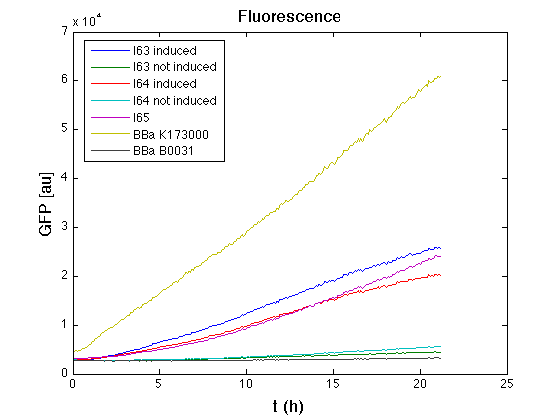
In GFP curve it's possible to appreciate that in induced I63, I64 and I65 GFP accumulation profile it's very similar and it's significantly different from that of negative control <partinfo>BBa_B0031</partinfo>. On the other hand not induced I63 and I64 show a profile very similar to the last one. These results show the right folding of the green fluorescent protein assembled downstream of the genetic circuit and that the inducible system works as expected. | In GFP curve it's possible to appreciate that in induced I63, I64 and I65 GFP accumulation profile it's very similar and it's significantly different from that of negative control <partinfo>BBa_B0031</partinfo>. On the other hand not induced I63 and I64 show a profile very similar to the last one. These results show the right folding of the green fluorescent protein assembled downstream of the genetic circuit and that the inducible system works as expected. | ||
Revision as of 07:19, 26 October 2010
|
|
||||||||||||||||||||||||||||||||
|
|
|
||||||||||||||||||||||||||||||
 "
"