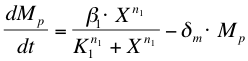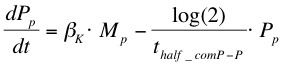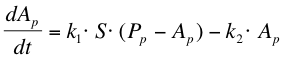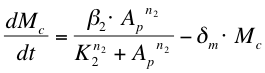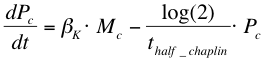Team:Groningen/Expression model
From 2010.igem.org
(→References) |
(→The model) |
||
| Line 24: | Line 24: | ||
=== The model === | === The model === | ||
| + | Our model describes the activation of ComP by ComX to the eventual formation of our chaplins. | ||
| + | |||
The activation of ComP-P expression by ComX (''X'') is modelled with a Hill’s equation. The degradation of the formed mRNA is taken into account. The equation describes the formation of ComP-P mRNA (''Mp'') in time. | The activation of ComP-P expression by ComX (''X'') is modelled with a Hill’s equation. The degradation of the formed mRNA is taken into account. The equation describes the formation of ComP-P mRNA (''Mp'') in time. | ||
Revision as of 10:52, 24 October 2010
Expression

Introduction
To form a biofilm with hydrophobic surface properties, expression of the hydrophobic proteins(in our case chaplins) should coincide with the late stages of biofilm formation. There are several reasons for this: mainly because not all bacteria participate in biofilm formation, there is significant cell differentiation prior to the formation of a biofilm. Also the expression of chaplins should not interfere with the growth speed and biofilm formation. The latter could be problematic due to the hydrophobic properties of the chaplins which might interfere with the formation of the extracellular matrix(ECM). We found that the ComXPA quorum sensing system and related gene expressions might suitable for this purpose.
ComXPA quorum sensing system
Quorum sensing in bacteria means that bacteria ‘talk’ to each other via signaling molecules. These molecules accumulate extracellularly as cultures grow to high cell densities. The response to these signaling molecules can result in dramatic changes in gene expression. In Bacillus subtilis a quorum sensing pathway named ComX-ComP-ComA is present which regulates the development of genetic competence. The ComX pheromone is a 10-amino-acid peptide which is secreted.
When ComX accumulates extracellularly, it stimulates the activity of a membrane bound receptor histidine kinase called ComP. ComP then autophosphorylates and activates the response regulator ComA by donating a phosphate molecule. The activated ComA functions as a transcriptional activator and activates the expression of 20 genes directly and the expression of another 150 genes is affected indirectly. The affected genes seem to have three different functions: the coordination of physiological changes involved in developmental pathways, the production of extracellular products and the enhancement of survival, growth and colonization. ComA directly activates the srfA operon which encodes enzymes needed for the production of the lipopeptide surfactin. The binding site for the activated ComA is known, the consensus sequence is a 12 bp palingdrome with a 4-5 bp spacer (TTGCGGNNNNCCGCAA). A DNA microarray study determined which genes were mostly activated by the active ComA (Comella et. al., 2005) . For our project we chose to link our chaplins to the promoter of the srfA gene which was most activated, which was srfAA.
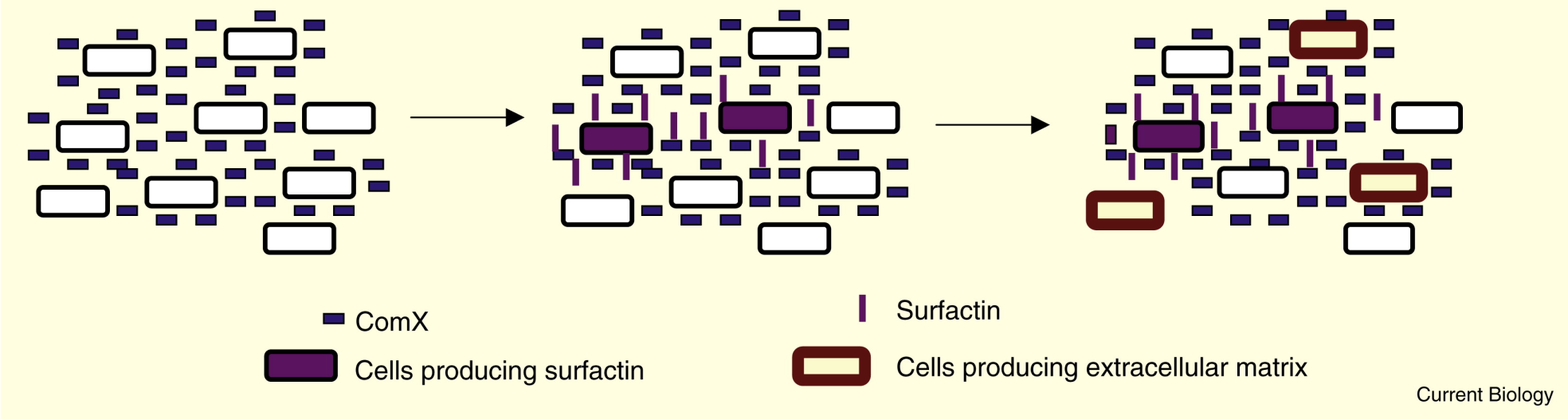
Surfactin and biofilm formation
Recently, it has been found that not all bacteria respond to the signaling molecules in the same way. It was shown that biofilm formation in Bacillus subtilis involves paracrine signaling (Lopez et al., 2009). Although most cells produce and secrete ComX, only a subpopulation of the cells is triggered to make surfactin. Surfactin serves as signaling molecule and the cells which are not able to make surfactin, respond to surfactin by making extracellular matrix components for the biofilm.
It is believed that the extracellular matrix interferes with the interaction between ComX and ComP and therefore prevents surfactin production in extracellular matrix producing cells. In a mutant which did not express extracellular matrix proteins, the surfactin expression was more than three times higher.
During our project we would like to explore the possibilities of expressing the chaplins directly as a extracellular matrix protein. This could be done by using the yqxM promotor, responsible for TasA expression, in both a TasA (null mutant) and a wild type strain.
The model
Our model describes the activation of ComP by ComX to the eventual formation of our chaplins.
The activation of ComP-P expression by ComX (X) is modelled with a Hill’s equation. The degradation of the formed mRNA is taken into account. The equation describes the formation of ComP-P mRNA (Mp) in time.
After the formation of mRNA, the protein ComP-P (Pp) will be formed which is modelled with the following equation:
The next reaction within the metabolism is the phosphorylation of ComA by ComP-P. The formation of ComA-P (Ap) is described by the following equation:
After phosphorylation, ComA-P is able to activate the expression of the gene that is behind the srfA promoter. This will be one of the chaplins in our case or GFP to test the system. Again, the Hill’s equation is used to model this process combined with the degradation of mRNA and the formation of the protein. The first equation shows the formation of mRNA of chaplins (Mc), the second equation describes the formation of the chaplin (Pc)
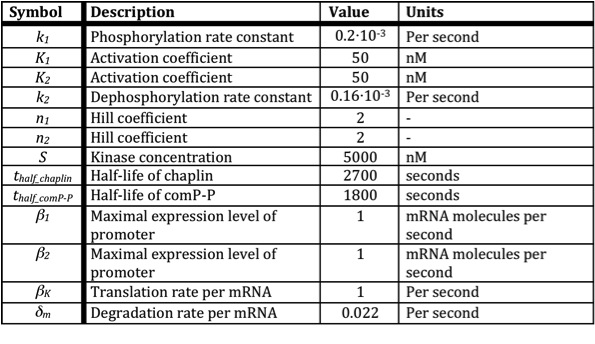
Below a table is shown with all the values of the used constants.
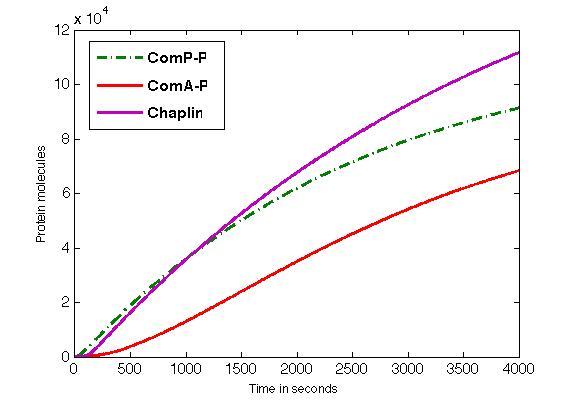
The figure shows the formation of ComP-P, ComA-P and chaplins in time.
References
Bischofs IB, Hug JA, Liu AW, Wolf DM, Arkin AP, Complexity in bacterial cell-cell communication: quorum signal integration and subpopulation signaling in the Bacillus subtilis phosphorelay, Proceedings of the National Academy of Sciences, 2009, 106 (16): 6459-64. http://www.ncbi.nlm.nih.gov/pubmed/19380751 (activationcoefficient K)
Mitrophanov AY, Groisman EA, Positive feedback in cellular control systems, Bioessays, 2008, (6): 542-55. http://www.ncbi.nlm.nih.gov.proxy-ub.rug.nl/pubmed/18478531 (Hills factor)
Comella N, Grossman AD., Conservation of genes and processes controlled by the quorum response in bacteria: characterization of genes controlled by the quorum-sensing transcription factor ComA in Bacillus subtilis, Molecular Microbiology (2005) 57 (4), 1159–1174. http://www.ncbi.nlm.nih.gov/pubmed/16091051
Leisner M, Kuhr JT, Rädler JO, Frey E, Maier B, Kinetics of genetic switching into the state of bacterial competence, Biophysical Journal, 2009, 96(3): 1178-88. http://www.ncbi.nlm.nih.gov/pubmed/19186153
Leisner M, Stingl K, Frey E, Maier B, Stochastic switching to competence, Current Opinion in Microbiology, 2008, 11(6):553-9. http://www.ncbi.nlm.nih.gov/pubmed/18955155
Leisner M, Stingl K, Rädler JO, Maier B, Basal expression rate of comK sets a 'switching-window' into the K-state of Bacillus subtilis, Molecular Microbiology, 2007, 63 (6): 1806-16. http://www.ncbi.nlm.nih.gov/pubmed/17367397
López D, Vlamakis H, Losick R, Kolter R., Paracrine signaling in a bacterium, Genes & Development, 2009 23(14):1631-8. http://www.ncbi.nlm.nih.gov/pubmed
Najafi SM, Harris DA, Yudkin MD, Properties of the phosphorylation reaction catalyzed by SpoIIAB that help to regulate sporulation of Bacillus subtilis, Journal of Bacteriology, 1997, 179 (17): 5628-5631. http://www.ncbi.nlm.nih.gov/pubmed/9287028 (phosphorylation rates and kinase concentration)
Roggiani M, Dubnau D., ComA, a phosphorylated response regulator protein of Bacillus subtilis, binds to the promoter region of srfA, Journal of Bacteriology 1993 175(10):3182-7. http://www.ncbi.nlm.nih.gov/pubmed/8387999
Shah IM, Dworkin J., Microbial interactions: bacteria talk to (some of) their neighbors, Current Biology, 2009 19(16): 689-91. http://www.ncbi.nlm.nih.gov/pubmed/19706277
Süel GM, Garcia-Ojalvo J, Liberman LM, Elowitz MB, An excitable gene regulatory circuit induces transient cellular differentiation, Nature, 2006, 440 (7083): 545-50. http://www.ncbi.nlm.nih.gov/pubmed/16554821
 "
"