Team:TU Delft/Project/alkane-degradation/parts
From 2010.igem.org
(→BBa_K398017 - Long-chain (C15-36) alkane conversion) |
(→BBa_K398018 - Long-chain alkanol conversion) |
||
| Line 26: | Line 26: | ||
==BBa_K398018 - Long-chain alkanol conversion== | ==BBa_K398018 - Long-chain alkanol conversion== | ||
The next step is catalyzed by a zinc-independent alcohol dehydrogenase from Bacillus thermoleovorans B23; a thermophilic bacterium [3-4]. The enzyme converts long-chain alkanols into their respective alkanals by oxidation of NADH. | The next step is catalyzed by a zinc-independent alcohol dehydrogenase from Bacillus thermoleovorans B23; a thermophilic bacterium [3-4]. The enzyme converts long-chain alkanols into their respective alkanals by oxidation of NADH. | ||
| + | [[Image:TUDelft_BBa_K398018]] | ||
==BBa_K398029 - Long-chain alkanal conversion== | ==BBa_K398029 - Long-chain alkanal conversion== | ||
Revision as of 16:39, 15 October 2010
Hydrocarbon conversion parts
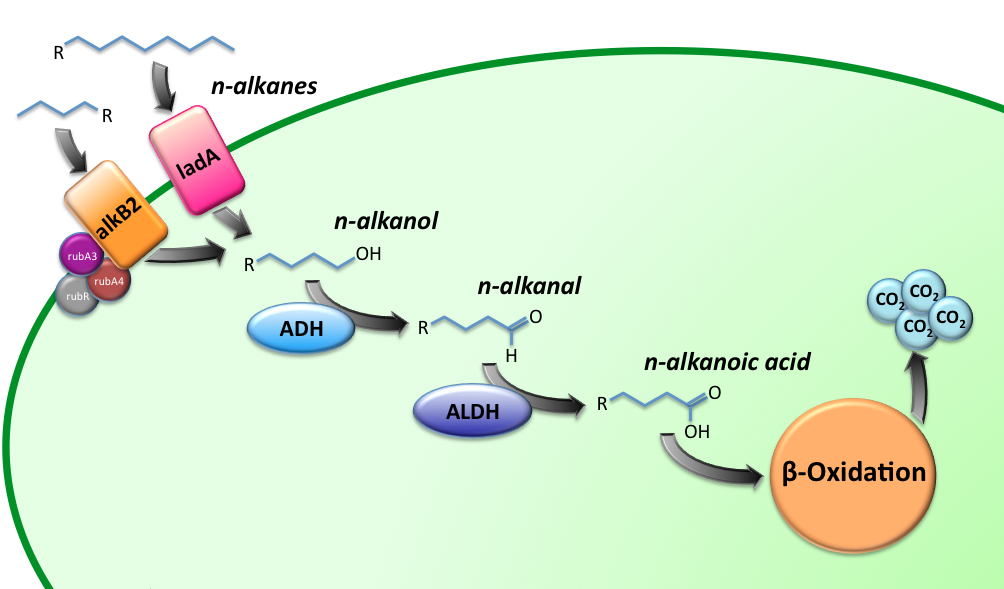
The Alkanivore E.coli strain has been designed to carry the genes required for the conversion of medium (C5-C13) and long chain (C15-C36) alkanes. A general scheme for the oxidation of the alkanes is illustrated in figure 1.
To create the alkane degradation constructs a number of genes encoding for alkane degradation enzymes were synthesized and combined with promoters and RBSs obtained from the BioBrick distribution plates. Combination of these genes resulted in the following BioBrick constructs (the intermediates have also been submitted to the registry).
BBa_K398014 - Medium-chain (C5-13) alkane conversion
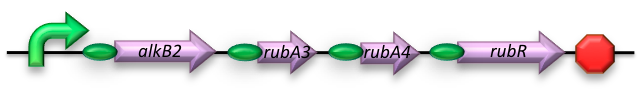
The short-chain hydrocarbon degrading BioBrick contains the AlkB gene cluster from ‘’Gordonia sp. TF6’’[1]. This will facilitate the initial step of the oxidation of C5-13 alkanes as well as that of C5-8 cycloalkanes. It is expected that the in-house mechanism of E.coli will be able to further degrade the products of this pathway. The gene cluster is formed by the genes encoding for alkB2 (alkane 1-monooxygenase), rubA3 (rubredoxin), rubA4 (rubredoxin) and rubR (rubredoxin reductase). AlkB2 is a non-haem diiron monooxygenase membrane protein, reported for several genus and species. This monooxygenase oxidizes n-alkanes to the respective n-alkanols and requires three soluble electron-transfer proteins, rubredoxin (rubA3 & rubA4) and rubredoxin reductase (rubR).
View this part in the [http://partsregistry.org/wiki/index.php?title=Part:BBa_K398014 parts registry]
BBa_K398017 - Long-chain (C15-36) alkane conversion
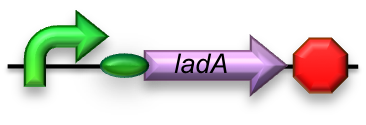
For the first step in the long-chain alkane degradation pathway the apoprotein ladA was implemented [2]; an alkane monooxygenase from ‘’Geobacillus thermodenitrificans’’ NG80-2. This protein facilitates the conversion of long-chain alkanes (from C15 up to at least C36) to their corresponding primary alcohols in the presence of flavin mononucleotide, oxygen and NADH.
BBa_K398018 - Long-chain alkanol conversion
The next step is catalyzed by a zinc-independent alcohol dehydrogenase from Bacillus thermoleovorans B23; a thermophilic bacterium [3-4]. The enzyme converts long-chain alkanols into their respective alkanals by oxidation of NADH. File:TUDelft BBa K398018
BBa_K398029 - Long-chain alkanal conversion
View these parts in the parts registry:
- [http://partsregistry.org/wiki/index.php?title=Part:BBa_K398017 ladA (BBa_K398017)]
- [http://partsregistry.org/wiki/index.php?title=Part:BBa_K398018 ADH (BBa_K398018)]
- [http://partsregistry.org/wiki/index.php?title=Part:BBa_K398019 ALDH (BBa_K398019)]
References
[1] Fujii, T., Narikawa, T., Takeda, K., Kato, J., Biotransformation of various alkanes using the Escherichia coli expressing an alkane hydroxylase system from Gordonia sp. TF6. Bioscience, biotechnology, and biochemistry, 68(10) 2171-2177 (2004)
[2] Liu Li, Xueqian Liu, Wen Yang, Feng Xu, Wei Wang, Lu Feng, Mark Bartlam, Lei Wang and Zihe Rao. Crystal Structure of Long-Chain Alkane Monooxygenase (LadA) in Complex with Coenzyme FMN: Unveiling the Long-Chain Alkane Hydroxylase. Journal of molecular biology, 376: 453–465 (2008)
[3] Tomohisa Kato, Asuka Miyanaga, Mitsuru Haruki, Tadayuki Imanaka, Masaaki Morikawa & Shigenori Kanaya. Gene Cloning of an Alcohol Dehydrogenase from Thermophilic Alkane-Degrading Bacillus thermoleovorans B23. Journal of Bioscience and Bioengineering 91(1):100-102 (2001)
[4] Tomohisa Kato, Asuka Miyanaga, Shigenori Kanaya, Masaaki Morikawa. Gene cloning and characterization of an aldehyde dehydrogenase from long-chain alkane-degrading Geobacillus thermoleovorans B23. Extremophiles 14:33-39 (2010)
 "
"