Team:UNIPV-Pavia/Calendar/July/settimana5
From 2010.igem.org
m (→July, 29th) |
m (→July, 29th) |
||
| (27 intermediate revisions not shown) | |||
| Line 11: | Line 11: | ||
<td valign="top"> | <td valign="top"> | ||
<table border="0" align="center" width="100%"><tr><td align="justify" valign="top" style="padding:20px"> | <table border="0" align="center" width="100%"><tr><td align="justify" valign="top" style="padding:20px"> | ||
| + | |||
| + | <table class="menu" border="0" width="100%"> | ||
| + | <tr> | ||
| + | <td align="center"> | ||
| + | [[Team:UNIPV-Pavia/Calendar/July/settimana1|Week 1]] | ||
| + | </td> | ||
| + | <td align="center" style="padding:0; height:20px"> | ||
| + | [[Team:UNIPV-Pavia/Calendar/July/settimana2|Week 2]] | ||
| + | </td> | ||
| + | <td align="center"> | ||
| + | [[Team:UNIPV-Pavia/Calendar/July/settimana3|Week 3]] | ||
| + | </td> | ||
| + | <td align="center"> | ||
| + | [[Team:UNIPV-Pavia/Calendar/July/settimana4|Week 4]] | ||
| + | </td> | ||
| + | <td align="center"> | ||
| + | [[Team:UNIPV-Pavia/Calendar/July/settimana5|Week 5]] | ||
| + | </td> | ||
| + | </tr> | ||
| + | </table> | ||
| + | <br> | ||
<html><p align="center"><font size="4"><b>JULY: WEEK 5</b></font></p></html><hr><br> | <html><p align="center"><font size="4"><b>JULY: WEEK 5</b></font></p></html><hr><br> | ||
| + | <html><a name="indice"/></html> | ||
==July, 26th== | ==July, 26th== | ||
| + | Ligation of I15_4C5=I15(E-P)+4C5(E-P) | ||
| + | |||
| + | We retrieved from our freezer I15-1 (quantified 34,2 ng/ul) and 4C5 (quantified 24,0 ng/ul) | ||
| + | Digestion E-P was performed: | ||
| + | |||
| + | |||
| + | {| border='1' | ||
| + | | ''Culture'' || ''Kind'' || ''Final reaction volume (ul) '' || ''DNA (ul)'' || ''H20 (ul)'' || ''Enzyme 1'' || ''Enzyme 2'' || ''Buffer H'' | ||
| + | |- | ||
| + | | I15-1 || Insert || 25 || 18,5 || 2 || 1 EcoRI || 1 PstI || 2,5 | ||
| + | |- | ||
| + | | pSB4C5 || Vector || 25 || 3,6 || 16,9 || 1 EcoRI || 1 PstI || 2,5 | ||
| + | |} | ||
| + | |||
| + | Digestions were incubated at 37°C for 3 hours. A medium 1% agarose gel was prepared. I15-1 didn't give good results, in fact many unwanted extra-bands were observed. For this reason, we decided not to perform ligation, but to sequence I15-1. An inoculum from glycerol stock for I15-1 was performed in 1ml LB+Amp, and incubated at 37°C 220rpm ON. Tomorrow I15-1 plasmide will be extracted with MiniPrep kit and I15-1 sample will be prepared for sequencing. | ||
| + | |||
| + | Today we also screened 3 contaminants of MC1061 transformed with RING, incubated yesterday | ||
| + | |||
| + | <table align='center'><tr><td> | ||
| + | [[Image:UNIPV10_I15_Extrabands.jpg|thumb|150px|center|4C5 (E-P) and I15-1 (E-P): extra-bands can be observed for I15-1]] | ||
| + | </td><td> | ||
| + | [[Image:UNIPV10_MC123_contaminants.jpg|thumb|150px|center|MC1-2-3 contaminants screening: no plasmid was observed]]</td></tr></table> | ||
| + | |||
| + | <div align="right"><small>[[#indice|^top]]</small></div> | ||
==July, 27th== | ==July, 27th== | ||
| + | Today we prepared I15-1 sample for sequencing. MiniPrep was performed on this culture and samples for both forward and reverse sequencing were prepared and sent to BMR genomics. | ||
| + | |||
| + | Our self-inducible parts assembly goes on: we are ready to co-trasform I14_4C5, I16_4C5, I17_4C5, I18_4C5 and I19_4C5 in T9002 competent strain (home made). | ||
| + | Since we noticed that our I7-3 and I8-5 glycerol stocks are not present in our freezer, we decided to prepare them today, starting from purified DNA (from MiniPrep) and tranforming it again in TOP10. | ||
| + | |||
| + | |||
| + | <table width='90%' border='1'> | ||
| + | <tr> | ||
| + | <td>'''Ligation name'''</td><td>'''E. coli strain''' </td><td> '''Resistance''' </td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td> | ||
| + | *I14_4C5-2= I14 (E-P) + pSB4C5 (E-P) | ||
| + | </td> | ||
| + | <td> | ||
| + | T9002 | ||
| + | </td> | ||
| + | <td>Amp 100+Cm 12,5</td> | ||
| + | </tr> | ||
| + | <tr><td> | ||
| + | *I16_4C5-1= I16 (E-P) + pSB4C5 (E-P) | ||
| + | </td> | ||
| + | <td> | ||
| + | T9002 | ||
| + | </td> | ||
| + | <td>Amp 100+Cm 12,5</td> | ||
| + | </tr> | ||
| + | <tr><td> | ||
| + | *I17_4C5-1= I17 (E-P) + pSB4C5 (E-P)</td> | ||
| + | <td> | ||
| + | T9002 | ||
| + | </td> | ||
| + | <td>Amp 100 + Cm 12,5</td> | ||
| + | </tr> | ||
| + | <tr><td> | ||
| + | *I18_4C5-1= I18 (E-P) + pSB4C5 (E-P)</td> | ||
| + | <td> | ||
| + | T9002 | ||
| + | </td> | ||
| + | <td>Amp 100 + Cm 12,5</td> | ||
| + | </tr> | ||
| + | <tr><td> | ||
| + | *I19_4C5-1= I19 (E-P) + pSB4C5 (E-P)</td> | ||
| + | <td> | ||
| + | T9002 | ||
| + | </td> | ||
| + | <td>Amp 100 + Cm 12,5</td> | ||
| + | </tr> | ||
| + | <tr><td> | ||
| + | *I7-3</td> | ||
| + | <td> | ||
| + | TOP10 | ||
| + | </td> | ||
| + | <td>Amp 100</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td> | ||
| + | *I8-5</td> | ||
| + | <td> | ||
| + | TOP10 | ||
| + | </td> | ||
| + | <td>Amp 100</td> | ||
| + | </tr> | ||
| + | </table> | ||
| + | |||
| + | MiniPrep was performed also on MC1 and MC2 (colonies grown on LB+Cm 12,5 for MC1061) | ||
| + | |||
| + | * MC1: 129,8 ng/ul, digested with HindIII | ||
| + | * MC2: 34 ng/ul, digested with HindIII | ||
| + | |||
| + | [[Image:UNIPV10_MC1_MC2_HindIII_and_unidigested_screening.jpg|thumb|150px|center|Screening for MC1 and MC2: digested with HindIII and undigested]] | ||
| + | |||
| + | <div align="right"><small>[[#indice|^top]]</small></div> | ||
==July, 28th== | ==July, 28th== | ||
| + | |||
| + | Today we received primers to modify PhaPs. We diluted primers to perform a PCR with them in order to mutagenize this BioBrick to get its prefix Standard/Silver compliant and suffix Silver compliant. | ||
| + | |||
| + | <div align="right"><small>[[#indice|^top]]</small></div> | ||
==July, 29th== | ==July, 29th== | ||
| - | We used our new synthesized primers to modify through PCR <partinfo>BBa_K208001</partinfo> phasin in order to create two new parts without stop codon | + | Today we performed PCR in order to amplify the phasin PhaP (<partinfo>BBa_K208001</partinfo>). Out primers are built to eliminate the stop codon from phasin and to give it the prefix and suffix we desire: |
| + | *PhaP with prefix compliant to the <html><a href="http://partsregistry.org/partsdb/scars.cgi"><b>10 Standard</b></a> and suffix compliant to the <a href="http://partsregistry.org/partsdb/scars.cgi"><b>Silver Standard</b></a></html> | ||
| + | *PhaP with prefix compliant to the <html><a href="http://partsregistry.org/partsdb/scars.cgi"><b>Silver Standard</b></a> and suffix compliant to the <a href="http://partsregistry.org/partsdb/scars.cgi"><b>Silver Standard</b></a></html> | ||
| + | |||
| + | We used our new synthesized primers to modify through PCR <partinfo>BBa_K208001</partinfo> phasin in order to create two new parts without stop codon but with Standard prefix and Silver suffix the first one, Silver prefix and suffix the second one. | ||
[[Image:UNIPV10_PCR_phasins.jpg|thumb|200px|center|Gel run for PCR-modified phasins]] | [[Image:UNIPV10_PCR_phasins.jpg|thumb|200px|center|Gel run for PCR-modified phasins]] | ||
| Line 45: | Line 172: | ||
*1 ul of MilliQ (negative control). | *1 ul of MilliQ (negative control). | ||
Transformed cells have been plated on Cm 12,5 ug/ml agar plates and incubated overnight at 37°C. | Transformed cells have been plated on Cm 12,5 ug/ml agar plates and incubated overnight at 37°C. | ||
| - | < | + | |
| - | + | <div align="right"><small>[[#indice|^top]]</small></div> | |
==July, 30th== | ==July, 30th== | ||
| Line 70: | Line 197: | ||
MC1061 competent cells don't replicate RING :) !!! | MC1061 competent cells don't replicate RING :) !!! | ||
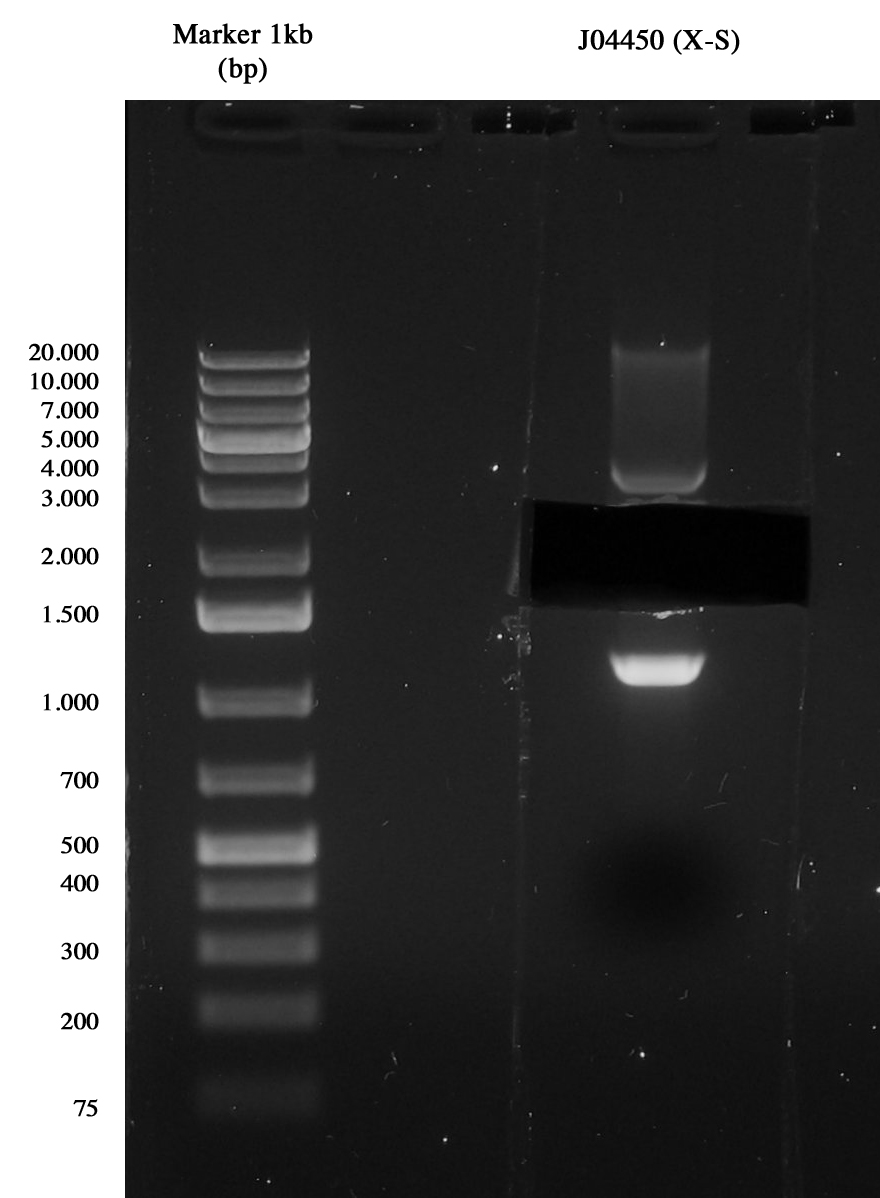
| - | Miniprep of J04450 to take the vector pSB1A3 | + | ---- |
| + | |||
| + | Miniprep of <partinfo>J04450</partinfo> to take the vector <partinfo>pSB1A3</partinfo>. | ||
3-hour digestion (X-S) and gel run to extract the backbone (~2157 bp). | 3-hour digestion (X-S) and gel run to extract the backbone (~2157 bp). | ||
| Line 87: | Line 216: | ||
*I20: Pha-10S-1 (X-S) + pSB1A3 (X-S) | *I20: Pha-10S-1 (X-S) + pSB1A3 (X-S) | ||
*I21: Pha-SS-1 (X-S) + pSB1A3 (X-S) | *I21: Pha-SS-1 (X-S) + pSB1A3 (X-S) | ||
| + | |||
| + | <div align="right"><small>[[#indice|^top]]</small></div> | ||
==July, 31st== | ==July, 31st== | ||
Transformation of I20 and I21 into ''E. coli'' DH5-alpha. Cells were plated on LB+Amp agar plates and grown overnight at 37°C. | Transformation of I20 and I21 into ''E. coli'' DH5-alpha. Cells were plated on LB+Amp agar plates and grown overnight at 37°C. | ||
| + | |||
| + | <div align="right"><small>[[#indice|^top]]</small></div> | ||
==August, 1st== | ==August, 1st== | ||
| Line 98: | Line 231: | ||
|} | |} | ||
| + | <div align="right"><small>[[#indice|^top]]</small></div> | ||
| + | <!-- table previous next week --> | ||
| + | <br><br> | ||
| + | <table border="0" width="100%" class="menu"> | ||
| + | <tr> | ||
| + | <td align="left">[[Team:UNIPV-Pavia/Calendar/July/settimana4| Previous week]]</td> | ||
| + | <td align="right">[[Team:UNIPV-Pavia/Calendar/August/settimana1| Next week]]</td> | ||
| + | </tr> | ||
| + | </table> | ||
| + | <!-- fine table previous next week --> | ||
| + | </td> | ||
| + | <td width="15%" align="right" valign="top"> | ||
| - | + | {{UNIPV-Pavia/menu_mesi}} | |
| - | + | </td> | |
| - | + | </tr> | |
| - | + | ||
| - | </td | + | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
</table> | </table> | ||
Latest revision as of 16:54, 24 October 2010
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 "
"