Week of 6/14
From 2010.igem.org
(Difference between revisions)
| (6 intermediate revisions not shown) | |||
| Line 37: | Line 37: | ||
*We then cut out each available band, purified, ligated, and finally transformed. | *We then cut out each available band, purified, ligated, and finally transformed. | ||
| + | |||
| + | ==June 15th== | ||
| + | |||
| + | |||
| + | *Flasks mini prepped | ||
| + | -J37033 (LuxR + RBS) | ||
| + | -C0261 (LuxI + RBS) | ||
| + | |||
| + | *All of the mini preps were digested with the following enzymes: | ||
| + | -J37033 (XP) | ||
| + | -C0261 (XP) | ||
| + | |||
| + | *The digests were put into a 37 degree Celsius water bath for an hour. The reaction was heat killed for 20 minutes in an 80 degree Celsius water bath. | ||
| + | |||
| + | *The two parts were then run on a gel in this order: Ladder, PFTV (another student's project), C0261, and J37033. | ||
| + | |||
<center>[[Image:6-15-10.jpg]]</center> | <center>[[Image:6-15-10.jpg]]</center> | ||
| + | |||
| + | |||
| + | |||
| + | *This time the parts came out very vibrantly. We extracted C0261, did the gel purification, ligated, and transformed. | ||
| + | |||
| + | ==June 16th== | ||
| + | |||
| + | |||
| + | *Flasks mini prepped | ||
| + | -R0010 (pLac) | ||
| + | -C0062 (LuxR) | ||
| + | |||
| + | *All of the mini preps were digested with the following enzymes: | ||
| + | -R0010 (XP) | ||
| + | -C0062 (XP) | ||
| + | |||
| + | *The digests were put into a 37 degree Celsius water bath for an hour. The reaction was then heat killed for 20 minutes in an 80 degree Celsius water bath. | ||
| + | |||
| + | *After the heat kill, an Agarose gel was run. The parts run on the gel were Ladder, R0010, and C0062. | ||
| + | |||
| + | |||
<center>[[Image:6-16-10.jpg]]</center> | <center>[[Image:6-16-10.jpg]]</center> | ||
| + | |||
| + | |||
| + | |||
| + | *After the gel was finished running, we extracted the necessary bands and purified them. | ||
| + | |||
| + | *We made notes of possible ligations: | ||
| + | -Lux Promoters <-> C0261 (LuxI) | ||
| + | -Lux Promoters <-> K274100 (Pigment) | ||
| + | -Constitutive Promoters <-> J37033 (Lux R) | ||
| + | -CI Promoters (K091107) <-> Lysis (K112022, K112808) | ||
| + | |||
| + | *Goal for June 17th: | ||
| + | -Ligate B0034 <-> C0051 | ||
| + | C0012 | ||
| + | C0062 | ||
| + | |||
| + | ==June 17th== | ||
| + | |||
| + | |||
| + | *Flasks mini prepped: | ||
| + | -J24679 | ||
| + | -K091107 | ||
| + | -R0061 | ||
| + | -J37033 | ||
| + | -K081007 | ||
| + | -LacI + RBS | ||
| + | |||
| + | *All of the mini preps were digested with the following enzymes: | ||
| + | -K081007 (XP) (Phosphatase) | ||
| + | -K091107 (SP) | ||
| + | -J37033 (XP) (Phosphatase) | ||
| + | -R0061 (SP) | ||
| + | -J37033 (PCR) (XP) | ||
| + | -C0261 (PCR) (XP) | ||
| + | |||
| + | *The digests were put into a 37 degree Celsius water bath for one hour. The reaction was then heat killed for 20 minutes in an 80 degree Celsius water bath. | ||
| + | |||
| + | *After the heat kill, an agarose gel was run in the order of Ladder, J37033 (PCR), K274100, C0261 (PCR), R0061, K091107, and then P, T, V (another project). | ||
<center>[[Image:6-17-10.jpg]]</center> | <center>[[Image:6-17-10.jpg]]</center> | ||
| + | |||
| + | |||
| + | |||
| + | *Once the gel was finished we extracted and purified the parts. | ||
| + | |||
| + | *We made competent cells, SOB media, and Tet plates. | ||
Latest revision as of 15:40, 5 August 2010
Contents |
June 14th
- Flasks mini prepped
-J37033 (LuxR + RBS) -C0261 (LuxI + RBS) -R0061 (Lux Promoter) -J23116 (Constitutive Promoter) -R0063 (Lux Promoter) -K145150 (Lux Promoter) -K091146 (Lux Promoter) -K274100 (Pigment) -J23108 (Constitutive Promoter)
- All of the mini preps were digested with the following:
-J37033 (XP) -C0261 (XP) -R0061 (ES) -J23116 (ES) -R0063 (ES) -K145150 (ES) -K091146 (ES) -K274100 (ES) -J23108 (ES)
- We ran the digest as usual: 1 hour in the water bath, 20 minutes heat kill. We did not phosphotase any of these as you can see all of these parts appear on the gel.
- The order was Ladder, J23108, K274100, K091146, K145150, R0063, J23116, R0061, C0261, and J37033.

- This gel came out well with the exception of K145150.
- We then cut out each available band, purified, ligated, and finally transformed.
June 15th
- Flasks mini prepped
-J37033 (LuxR + RBS) -C0261 (LuxI + RBS)
- All of the mini preps were digested with the following enzymes:
-J37033 (XP) -C0261 (XP)
- The digests were put into a 37 degree Celsius water bath for an hour. The reaction was heat killed for 20 minutes in an 80 degree Celsius water bath.
- The two parts were then run on a gel in this order: Ladder, PFTV (another student's project), C0261, and J37033.
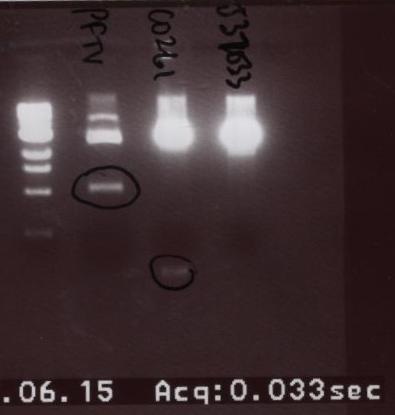
- This time the parts came out very vibrantly. We extracted C0261, did the gel purification, ligated, and transformed.
June 16th
- Flasks mini prepped
-R0010 (pLac) -C0062 (LuxR)
- All of the mini preps were digested with the following enzymes:
-R0010 (XP) -C0062 (XP)
- The digests were put into a 37 degree Celsius water bath for an hour. The reaction was then heat killed for 20 minutes in an 80 degree Celsius water bath.
- After the heat kill, an Agarose gel was run. The parts run on the gel were Ladder, R0010, and C0062.
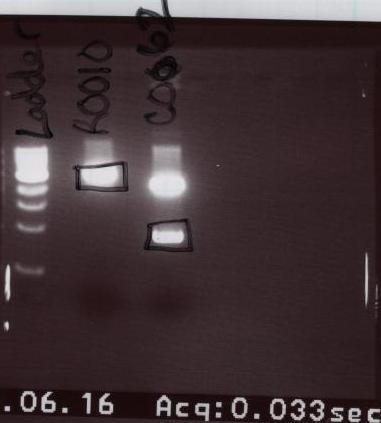
- After the gel was finished running, we extracted the necessary bands and purified them.
- We made notes of possible ligations:
-Lux Promoters <-> C0261 (LuxI) -Lux Promoters <-> K274100 (Pigment) -Constitutive Promoters <-> J37033 (Lux R) -CI Promoters (K091107) <-> Lysis (K112022, K112808)
- Goal for June 17th:
-Ligate B0034 <-> C0051
C0012
C0062
June 17th
- Flasks mini prepped:
-J24679 -K091107 -R0061 -J37033 -K081007 -LacI + RBS
- All of the mini preps were digested with the following enzymes:
-K081007 (XP) (Phosphatase) -K091107 (SP) -J37033 (XP) (Phosphatase) -R0061 (SP) -J37033 (PCR) (XP) -C0261 (PCR) (XP)
- The digests were put into a 37 degree Celsius water bath for one hour. The reaction was then heat killed for 20 minutes in an 80 degree Celsius water bath.
- After the heat kill, an agarose gel was run in the order of Ladder, J37033 (PCR), K274100, C0261 (PCR), R0061, K091107, and then P, T, V (another project).

- Once the gel was finished we extracted and purified the parts.
- We made competent cells, SOB media, and Tet plates.
 "
"