Team:UCL London/Approach
From 2010.igem.org
(→Ultra-Scale Down & Automation) |
(→Batch & Fed-batch) |
||
| (8 intermediate revisions not shown) | |||
| Line 35: | Line 35: | ||
'''Inoculation opening''': Feed line for which inoculum is transferred. | '''Inoculation opening''': Feed line for which inoculum is transferred. | ||
| + | |||
| + | ===Batch & Fed-batch=== | ||
| + | |||
| + | Considering a system to be any matter identified for investigation, we must first set apart the system and the surrounding which is technically the "remainder of the universe". The system and the "remainder of the universe" are separated by the system boundaries. | ||
| + | |||
| + | ''In a fermentation process, the system consists of everything inside the fermenter. Fermentations are ideally performed in closed systems with the objective of reducing interaction with the surrounding to eliminate any chances of contamination. If the system is completely closed, with nothing entering and nothing exiting the fermenter throughout the whole process, the process is called a batch. In other words, a batch process is one where all the materials are added at the start of the process, then the system is closed and product is removed only when the process is complete. Because batch processes means an early depletion of nutrients required producing a certain product, a more efficient way of exploiting the cells ability to produce was realised. This entails the addition of material into the fermenter as the process is ongoing. These types of processes are by far the most common in the pharmaceutical industry and are called Fed-Batch.'' | ||
| + | |||
| + | A batch culture is in essence a closed system with a fixed culture volume in which the cells grow to maximum cell density. This growth is highly dependent on the following factors: | ||
| + | |||
| + | • Medium nutrients | ||
| + | |||
| + | • Product toxicity | ||
| + | |||
| + | • Waste product toxicity | ||
| + | |||
| + | Classic kinetics is followed by the cells in which a log phase consisting of rapid proliferation takes place where some protein product is expressed and a stationary phase where the amount of cells does not change and where other products are produced. If the desired product is produced in the log phase, it can be prolonged by manipulating the growth conditions but only for a very limited time, and if the desired amount is not produced that quickly, it will be reasonable to choose another culture method. When the batch culture is terminated, the entire batch is harvested in one operation. | ||
| + | |||
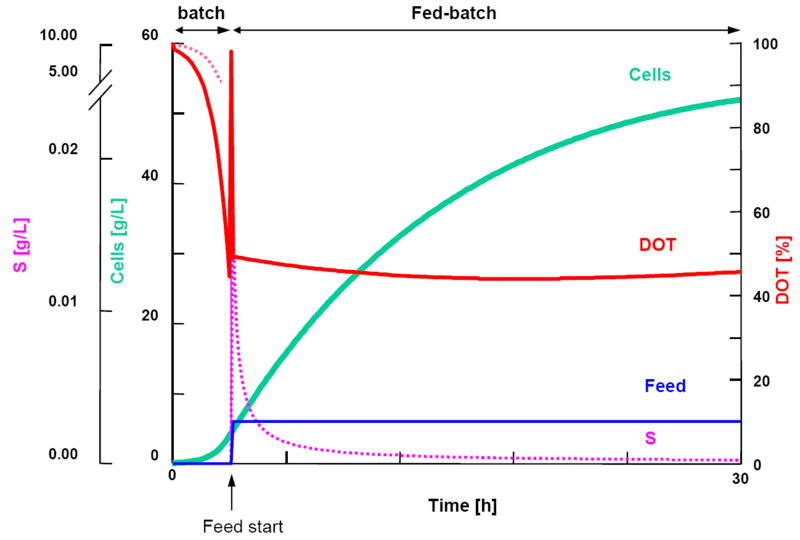
| + | [[Image:UCL-batchfedbatch.png|900px|right]] | ||
| + | |||
| + | In terms of a fed-batch mode, medium is added in fixed volumes throughout the process thus increasing the volume of the cell culture with time. Neither cells nor medium leave the bioreactor in order for sugar levels to be be kept more or less constant for a long time and it is possible to switch from one substrate to another thus rendering the use of inducible promoters possible. The process is usually performed at low growth rates, the key control parameter being the feed rate whose upper limit is dictated by the oxygen transfer limit and cooling strategies available. The feed rate can be subjected to feedback control strategies using for instance measurement of the glucose concentration, dissolved oxygen (DO), biomass production or heat generation. | ||
| + | |||
| + | “Batch to Fed-Batch mode” allows a transition between the two phases in which once the primary carbon source has been depleted, the oxygen concentration in the fermenter decreases as more cells have proliferated in the log phase. Once this point has been reached another carbon source is used to provide the interface of fed-batch mode in which the cells continue in stationary phase. | ||
==Dissolved Oxygen Tension (DOT) levels during fermentation== | ==Dissolved Oxygen Tension (DOT) levels during fermentation== | ||
| Line 64: | Line 86: | ||
== Directed Evolution == | == Directed Evolution == | ||
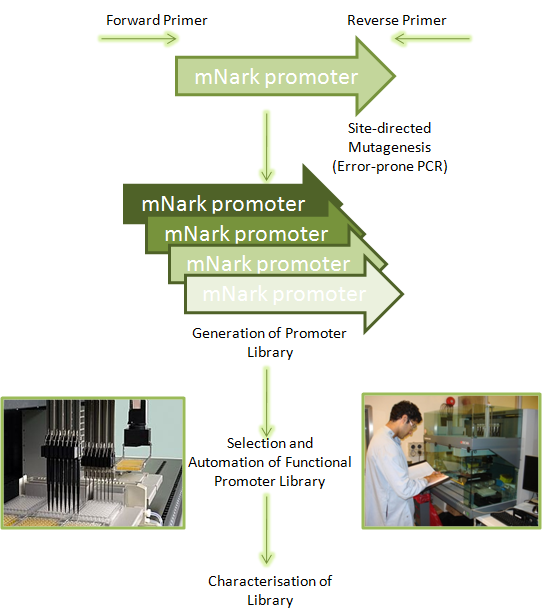
| - | [[Image: | + | [[Image:Muta-Evo.png|450px|right]] |
The ideal approach to implementing Project Hypoxon in biopharmaceutical manufacture is to improve its robustness and sensitivity as a genetic network. This relays the vision that the ''E. coli'' will perform consistently to the point in time required in fermentation and express the medicine required. At UCL’s Biochemical engineering department, there is opportunity to carry out Directed Evolution through the concept of promoter engineering combined with a suitable selection strategy to optimise the regulatory properties of the hypoxia sensing promoter (mNark). | The ideal approach to implementing Project Hypoxon in biopharmaceutical manufacture is to improve its robustness and sensitivity as a genetic network. This relays the vision that the ''E. coli'' will perform consistently to the point in time required in fermentation and express the medicine required. At UCL’s Biochemical engineering department, there is opportunity to carry out Directed Evolution through the concept of promoter engineering combined with a suitable selection strategy to optimise the regulatory properties of the hypoxia sensing promoter (mNark). | ||
| Line 100: | Line 122: | ||
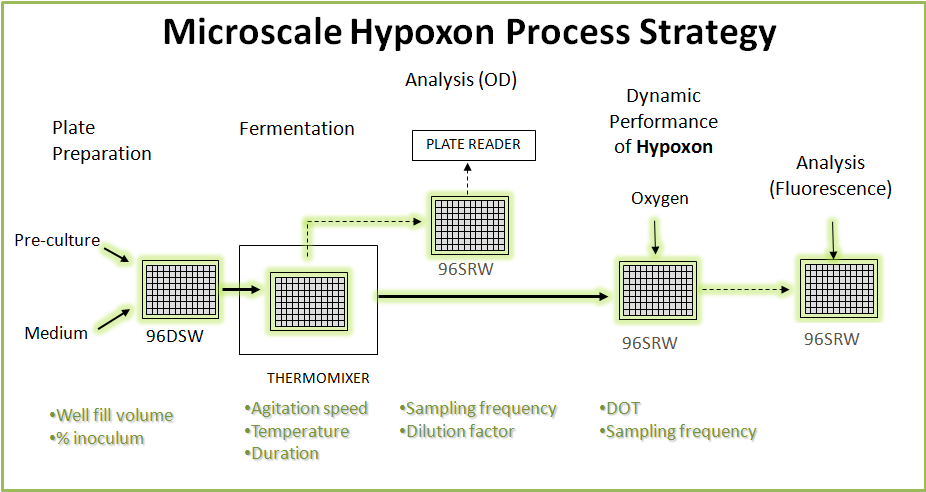
[[Image:strat.png|600px|center]] | [[Image:strat.png|600px|center]] | ||
| - | + | It the capability of intertwining research at various scales that set's the department aside as the world's elite in terms of Bioprocess development. The above exemplifies a bioconversion that has been moulded to as a provisional strategy for the automated characterisation of the mutated promoters. | |
| - | [[Image:Scale-down.JPG| | + | [[Image:Scale-down.JPG|400px|left]][[Image:Microplate.JPG|400px|left]] |
| + | [[Image:Plate1.JPG|400px|left]][[Image:MicroScreen.JPG|400px|left]] | ||
<div class='clearfix'> </div> | <div class='clearfix'> </div> | ||
<div class='clearfix'> </div> | <div class='clearfix'> </div> | ||
Latest revision as of 23:50, 27 October 2010
Biochemical Engineering
Project Hypoxon stems from a vision that cells can be programmed to detect external changes in their immediate environment and have a corresponding response. This idea is intrisic to moving away from manual operation in the manufacture of Biopharmaceuticals to more controlled techniques. The project is based in the world of Biochemical Engineering and Bioprocessing which is unfamiliar territory for most, yet its proven and ongoing growth in the treatment of disease through Biopharmaceuticals sets it aside as an advancing industry.
In our department, we believe that extraordinary advances in the life sciences have great potential to improve our quality of life, this is ultimately achieved through better medicines and a cleaner environment. We provide the foundations for the transforming of such extraordinary discoveries into products and processes leading to the improvement of health and welfare state of the world.
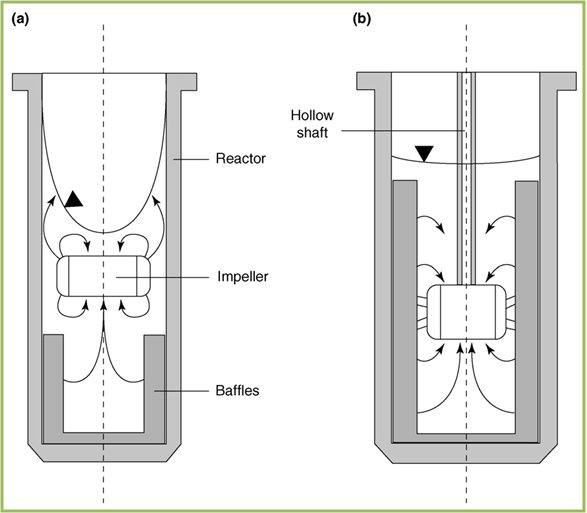
Fermenters
UCL iGEM 2010 will be implementing the use of its sophisticated class of 1 litre fermenters for the first time this year. In the biopharmaceutical industry, protein expression takes place on a much larger scale as a lot more protein product has to be produced. The recombinant E. coli cells are cultured under the controlled environment within the "fermenters" reaching volumes of around 10,000 L. A medium is firstly created containing vital chemicals that ensure that the cells have the resources to maintain growth.
The diagram adjacent exhibits one of the 1 litre fermenters that will be used to for testing and analysis of specific strains. The main components of the fermenter that encompass a range of scales have been labelled for further understanding:
Main Fermentation Chamber: This is where our E. coli cells are grown and where the fermentation process takes place.
Impellers: Responsible for ensuring homogeneous conditions are present for effective spread of oxygen and resources.
Temperature probe: Used to measure the temperature of the fermentation broth.
pH probe: Used to detect any changes in the pH.
Base and Acid: Used as resevoirs to adjust the pH of the vessel if conditions change.
Inlet air: Air is piped directly to a sparger to deliver oxygen for the growth of the E. coli cells
Outlet air: To remove outlet gases produced during the fermentation.
Sampling tube: Samples of the medium can be taken at various intervals and then OD or stored.
Inoculation opening: Feed line for which inoculum is transferred.
Batch & Fed-batch
Considering a system to be any matter identified for investigation, we must first set apart the system and the surrounding which is technically the "remainder of the universe". The system and the "remainder of the universe" are separated by the system boundaries.
In a fermentation process, the system consists of everything inside the fermenter. Fermentations are ideally performed in closed systems with the objective of reducing interaction with the surrounding to eliminate any chances of contamination. If the system is completely closed, with nothing entering and nothing exiting the fermenter throughout the whole process, the process is called a batch. In other words, a batch process is one where all the materials are added at the start of the process, then the system is closed and product is removed only when the process is complete. Because batch processes means an early depletion of nutrients required producing a certain product, a more efficient way of exploiting the cells ability to produce was realised. This entails the addition of material into the fermenter as the process is ongoing. These types of processes are by far the most common in the pharmaceutical industry and are called Fed-Batch.
A batch culture is in essence a closed system with a fixed culture volume in which the cells grow to maximum cell density. This growth is highly dependent on the following factors:
• Medium nutrients
• Product toxicity
• Waste product toxicity
Classic kinetics is followed by the cells in which a log phase consisting of rapid proliferation takes place where some protein product is expressed and a stationary phase where the amount of cells does not change and where other products are produced. If the desired product is produced in the log phase, it can be prolonged by manipulating the growth conditions but only for a very limited time, and if the desired amount is not produced that quickly, it will be reasonable to choose another culture method. When the batch culture is terminated, the entire batch is harvested in one operation.
In terms of a fed-batch mode, medium is added in fixed volumes throughout the process thus increasing the volume of the cell culture with time. Neither cells nor medium leave the bioreactor in order for sugar levels to be be kept more or less constant for a long time and it is possible to switch from one substrate to another thus rendering the use of inducible promoters possible. The process is usually performed at low growth rates, the key control parameter being the feed rate whose upper limit is dictated by the oxygen transfer limit and cooling strategies available. The feed rate can be subjected to feedback control strategies using for instance measurement of the glucose concentration, dissolved oxygen (DO), biomass production or heat generation.
“Batch to Fed-Batch mode” allows a transition between the two phases in which once the primary carbon source has been depleted, the oxygen concentration in the fermenter decreases as more cells have proliferated in the log phase. Once this point has been reached another carbon source is used to provide the interface of fed-batch mode in which the cells continue in stationary phase.
Dissolved Oxygen Tension (DOT) levels during fermentation
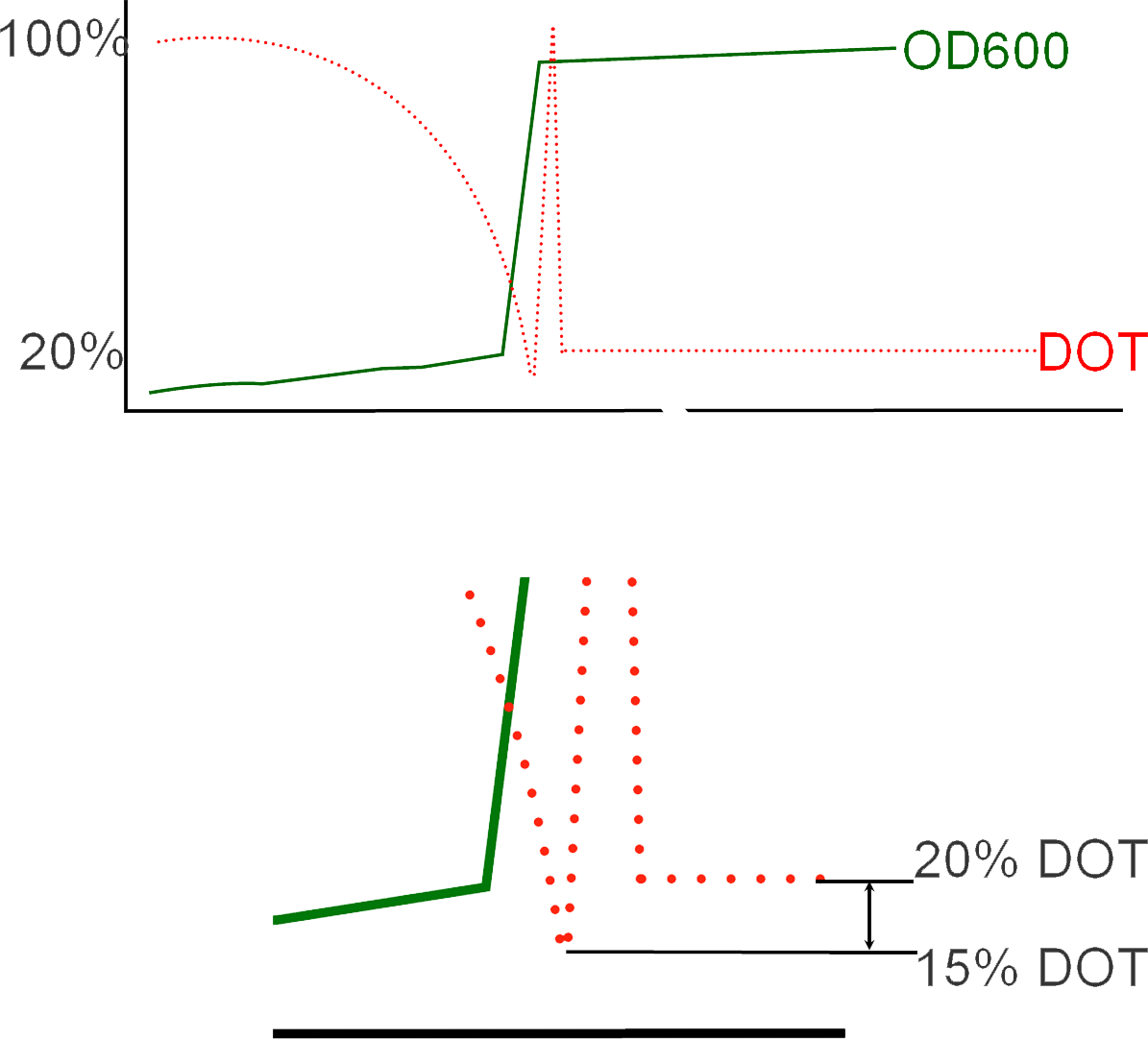
Initially during a fermentation run, DOT levels in the batch fermenter are observed with a gradual decreasing as a result of the E.Coli cells populating in growth. Over time, the DOT in the vessel will fall below 20% in which a critical point in the fermentation is reached as such low concentrations of oxygen will result in cellular death and loss of batch material. This rectified by adding the reagent IPTG (Isopropyl β-D-1-thiogalactopyranoside) in order to induce protein expression. This momentary measurement of low oxygen levels results in what is known in industry as an "Oxygen Spike" and marks the transition of the process from Batch to Fed-Batch in which there is a consistent addition of glycerol to sustain the cells during protein expression. The resultant shift from cellular growth to protein expression can be observed in the immediate increase in OD values subsequent to inoculation.
Protein Expression
E. coli one of the most widely applied in-vivo expression systems. The reason for its wide application is the fact that it is so well developed and the E. coli genome is fully developed and well understood. This can be emphasised by the ease in biotechnology for a DNA sequence for a protein of interest could be inserted into a high copy-number plasmid containing the lac promoter, which is then transformed into the bacterium Escherichia coli. Addition of IPTG (a lactose analog) activates the lac promoter and causes the bacteria to express the protein of interest.
One technique of insuring high levels of a protein is to clone the gene downstream of a well-characterized regulated promoter. Project Hypoxon will exploit the very widely used tac promoter/operator (Ptac) expression system. Ptac is a strong hybrid promoter composed of the -35 region of the trp promoter and the -10 region of the lacUV5 promoter/operator. As a strong hybrid promoter, it is repressed by the LacI protein and on addition of IPTG, the lacI repressser is inactivated. This inactivtation breaks the strong repression of Ptac resulting in expression of pTAC. It has been shown that high expressions of Ptac is directly proportional to the concentration of IPTG added. Therefore, By varying the IPTG concentration the amount of gene product cloned downstream from Ptac can be varied over several orders of magnitude.
Artists' Animation
In order to animate and summarise the aims of the ideal fermentation unit operation, a simple video has been produced thanks to the efforts of our artists.
The green blocks represent DOT levels in the fermenter, the red-blue represents the rate of protein expression subsequent ( 2 representing the positive feedback loop of 2 promoters and 2 promoter activators) and yellow being the activation of the hypoxia sensitive promoter due to the reduction of the DOT level below the threshold of around 20%.
The animations portrays the initial slow then rapid fall of DOT concentration which induces hypoxia as emphasised by the sudden appearance then dissappearance of the yellow lego bricks. As a result the genetic circuit as activated as the rate of protein expression increases. A subsequent increase in DOT levels is also observed. These set of rapid changes in DOT levels is known as the as aforementioned "oxygen spike" which is highly prevalent phenomenon in literature.
Directed Evolution
The ideal approach to implementing Project Hypoxon in biopharmaceutical manufacture is to improve its robustness and sensitivity as a genetic network. This relays the vision that the E. coli will perform consistently to the point in time required in fermentation and express the medicine required. At UCL’s Biochemical engineering department, there is opportunity to carry out Directed Evolution through the concept of promoter engineering combined with a suitable selection strategy to optimise the regulatory properties of the hypoxia sensing promoter (mNark).
By isolating mutants that could be prospectively induced under less stringent oxygen conditions than the wild type promoter, induction in industrial fermentations can move even further from the transition between batch and fed-batch to automated changes of oxygen concentration. Other potential improvements could be an improved overall efficiency in terms of homogenous expression in the fermenter where oxygen gradients could occur. Strategies in terms of implementing Project Hypoxon can be configured using the department’s state of the art Ultra-Scale down technology and exciting automated machinery.
Ultra-Scale Down & Automation
The development of industrial-scale bioprocesses is a costly and time consuming exercise and generally occurs at the point where speed of process development is key to getting the product through validation to market. This is made more challenging by the difficulty in scaling up many bioprocesses due to the complex interactions between the process conditions and the material. Scaling up is simply the expansion of how much protein is produced using the same process flow sheet.
The Bioprocess complexity is further increased when the whole process considered in terms of growing the E. coli cells and purifying into the final product due multiple interacting variables such as protein shear damage. Microscale approaches can in principle be applied to the study of each unit operation (filtration, centrifugation, etc) in a whole bioprocess sequence. The information obtained could either directly mimic larger scale performance or provide insight into key scale-up issues. As shown below, the transition between scales is quite large and the consumption of resources especially during process development decreases to a great extent by analysing performances. Being such an avant-garde approach, it dwells on a key feature of micro-biochemical engineering in that predictions of large scale performance do not demand scalar (making fermenters or columns bigger) but environment analysis is needed.
Key parameters include: Protein shear sensitivity and complex colloid content
Recent advances in scale down technology spearheaded at UCL have progressed to automated microwell-based methods which allow software capabilities to enhance the capacity to iterate between conditions. In terms of Project Hypoxon, hundreds of fermentations can be run simultaneously at microscale in these deep-wells (as shown adjacent) at volumes of 0.5µL (as opposed to hundreds) and run to analyse the dynamic performance of the Hypoxon circuit. These wells act as miniature reactors and can be further developed as shown at UCL where in one case miniature gas sparging impellers have been used to assist with oxygen transfer and sustain E. coli growth.
Ultra-Scale down Automation Summary:
• Allows collection of quantitative data early in the drug development process
• Allows screening and test of large libraries
• Allows the creation of a fully automated sequence
• Saves raw material, costs and time
• Allows use of repetitive procedures
It the capability of intertwining research at various scales that set's the department aside as the world's elite in terms of Bioprocess development. The above exemplifies a bioconversion that has been moulded to as a provisional strategy for the automated characterisation of the mutated promoters.


 "
"









 Twitter
Twitter Facebook
Facebook UCL
UCL Flickr
Flickr YouTube
YouTube