Team:UNIPV-Pavia/Parts/Characterization/RebExistingParts
From 2010.igem.org
m (→BBa_K300003 - Phasin (PhaP) - internal domain) |
|||
| (5 intermediate revisions not shown) | |||
| Line 32: | Line 32: | ||
=Improved Parts: list= | =Improved Parts: list= | ||
| - | * [[Team:UNIPV-Pavia/Parts/Characterization/RebExistingParts #BBa_K300002 - Phasin (PhaP) - head domain|BBa_K300002 - Phasin (PhaP) - head domain]] | + | * [[Team:UNIPV-Pavia/Parts/Characterization/RebExistingParts #BBa_K300002 - Phasin (PhaP) - head domain|BBa_K300002 - Phasin (PhaP) - head domain - improvement of BBa_K208001]] |
| - | * [[Team:UNIPV-Pavia/Parts/Characterization/RebExistingParts #BBa_K300003 - Phasin (PhaP) - internal domain|BBa_K300003 - Phasin (PhaP) - internal domain]] | + | * [[Team:UNIPV-Pavia/Parts/Characterization/RebExistingParts #BBa_K300003 - Phasin (PhaP) - internal domain|BBa_K300003 - Phasin (PhaP) - internal domain - improvement of BBa_K208001]] |
<br> | <br> | ||
| Line 43: | Line 43: | ||
=Phasins= | =Phasins= | ||
| - | Polyhydroxyalkanoates (PHAs) are polyoxoesters that are produced by | + | Polyhydroxyalkanoates (PHAs) are polyoxoesters that are produced by several bacteria and that accumulate as intracellular granules. Phasins are proteins that can bind these granules. |
| - | + | The following parts are derived from the phaP gene of ''Ralstonia eutropha'', which encodes for a phasin, engineered without the stop codon in order to support protein fusions as a head/internal domain. | |
Phasins can be used as affinity tags for a target protein, which can bind PHA granules allowing this way protein purification. | Phasins can be used as affinity tags for a target protein, which can bind PHA granules allowing this way protein purification. | ||
In literature [Banki MR et al., 2005] it has been shown that affinity tags composed by phasins assembled in tandem can increase the affinity with PHA. | In literature [Banki MR et al., 2005] it has been shown that affinity tags composed by phasins assembled in tandem can increase the affinity with PHA. | ||
| + | |||
| + | The aim of our improvement of phasin already present in the Registry (<partinfo>BBa_K208001</partinfo>) was to provide a head and an internal protein domain useful to build fusion proteins. | ||
==<partinfo>BBa_K300002</partinfo> - Phasin (PhaP) - head domain== | ==<partinfo>BBa_K300002</partinfo> - Phasin (PhaP) - head domain== | ||
| Line 130: | Line 132: | ||
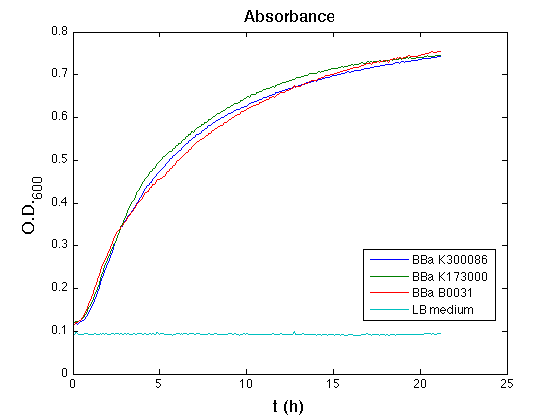
All cell cultures showed a similar growth curve; doubling time was computed as described [[Team:UNIPV-Pavia/Parts/Characterization#Doubling_time_evaluation|here]] in order to have informations about the burden due to the synthesis of such fusion proteins. It's possible to see that all doubling time are very similar; it's possible to assert that the expression of these BioBrick parts doesn't cause abnormal stress to cells. | All cell cultures showed a similar growth curve; doubling time was computed as described [[Team:UNIPV-Pavia/Parts/Characterization#Doubling_time_evaluation|here]] in order to have informations about the burden due to the synthesis of such fusion proteins. It's possible to see that all doubling time are very similar; it's possible to assert that the expression of these BioBrick parts doesn't cause abnormal stress to cells. | ||
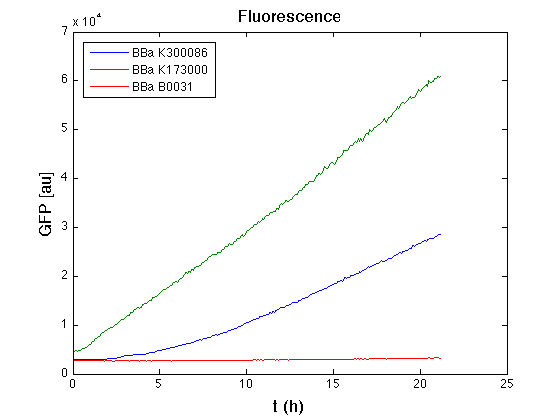
| - | From GFP curve it's possible to appreciate that in <partinfo>BBa_K300086</partinfo> GFP accumulation it's significantly different from that of negative control <partinfo>BBa_B0031</partinfo>. This result that the green fluorescent protein assembled downstream of the genetic circuit is correctly folded. | + | From GFP curve it's possible to appreciate that in <partinfo>BBa_K300086</partinfo> GFP accumulation it's significantly different from that of negative control <partinfo>BBa_B0031</partinfo>. This result shows that the green fluorescent protein assembled downstream of the genetic circuit is correctly folded. |
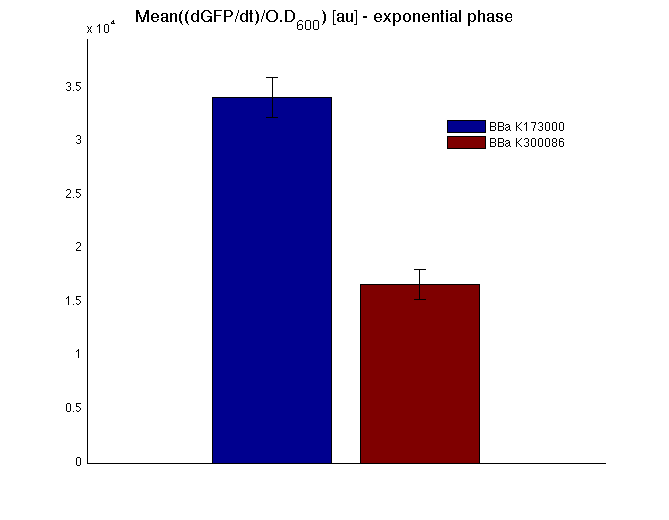
The mean protein synthesis rate was also computed over the growth exponential phase, showing an appreciable GFP production rate that is about a half of the positive control. | The mean protein synthesis rate was also computed over the growth exponential phase, showing an appreciable GFP production rate that is about a half of the positive control. | ||
Latest revision as of 03:25, 28 October 2010
|
|
|||||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||
 "
"