Team:NYMU-Taipei/Experiments/Riboswitch
From 2010.igem.org
(Difference between revisions)
(→2010.10.15) |
(→2010.10.22) |
||
| (28 intermediate revisions not shown) | |||
| Line 14: | Line 14: | ||
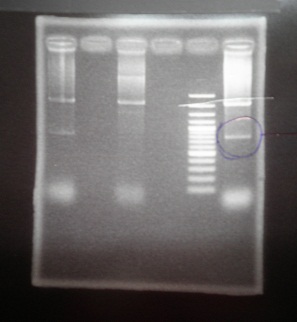
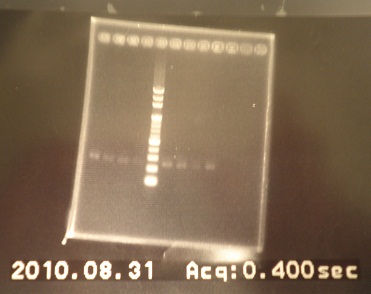
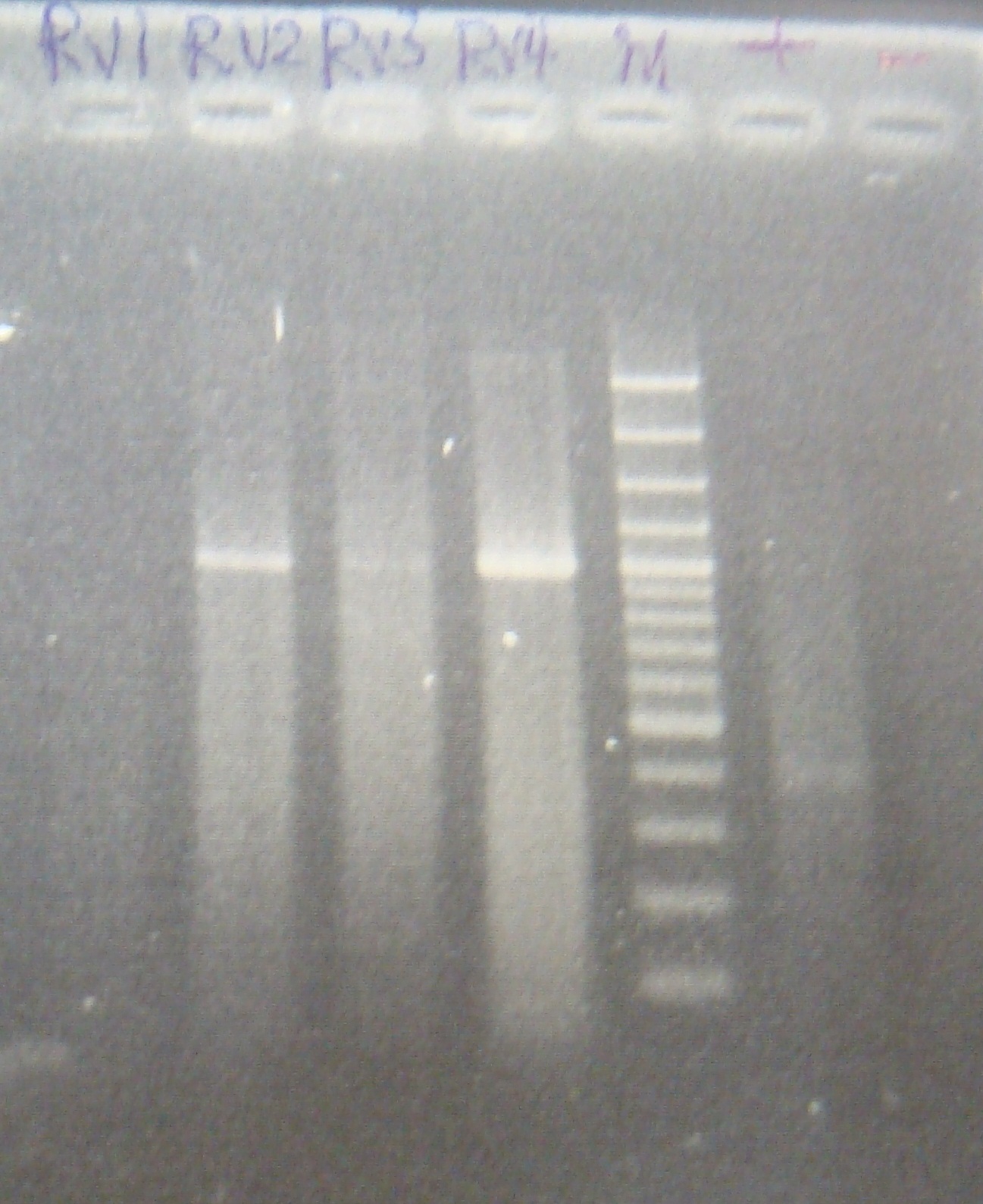
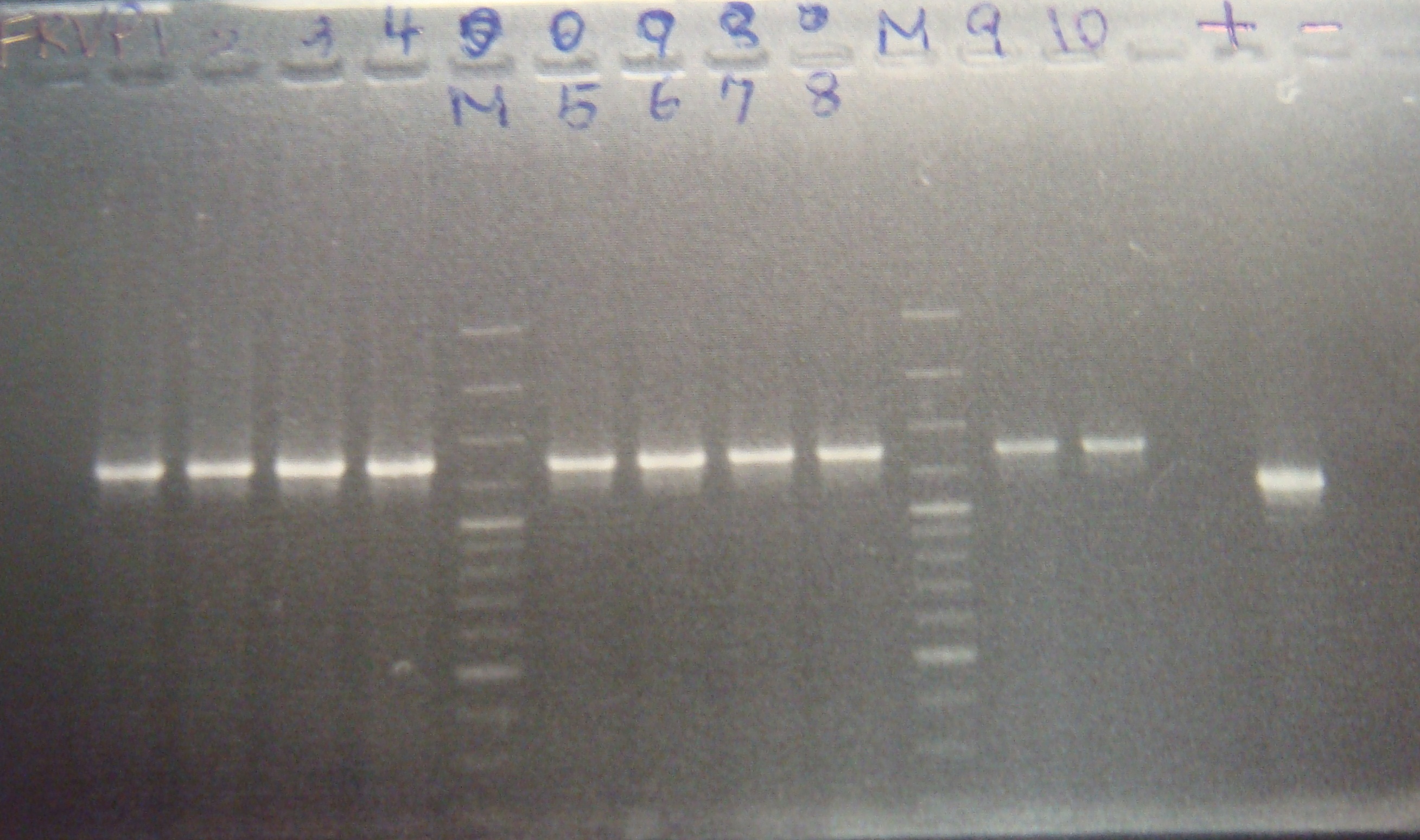
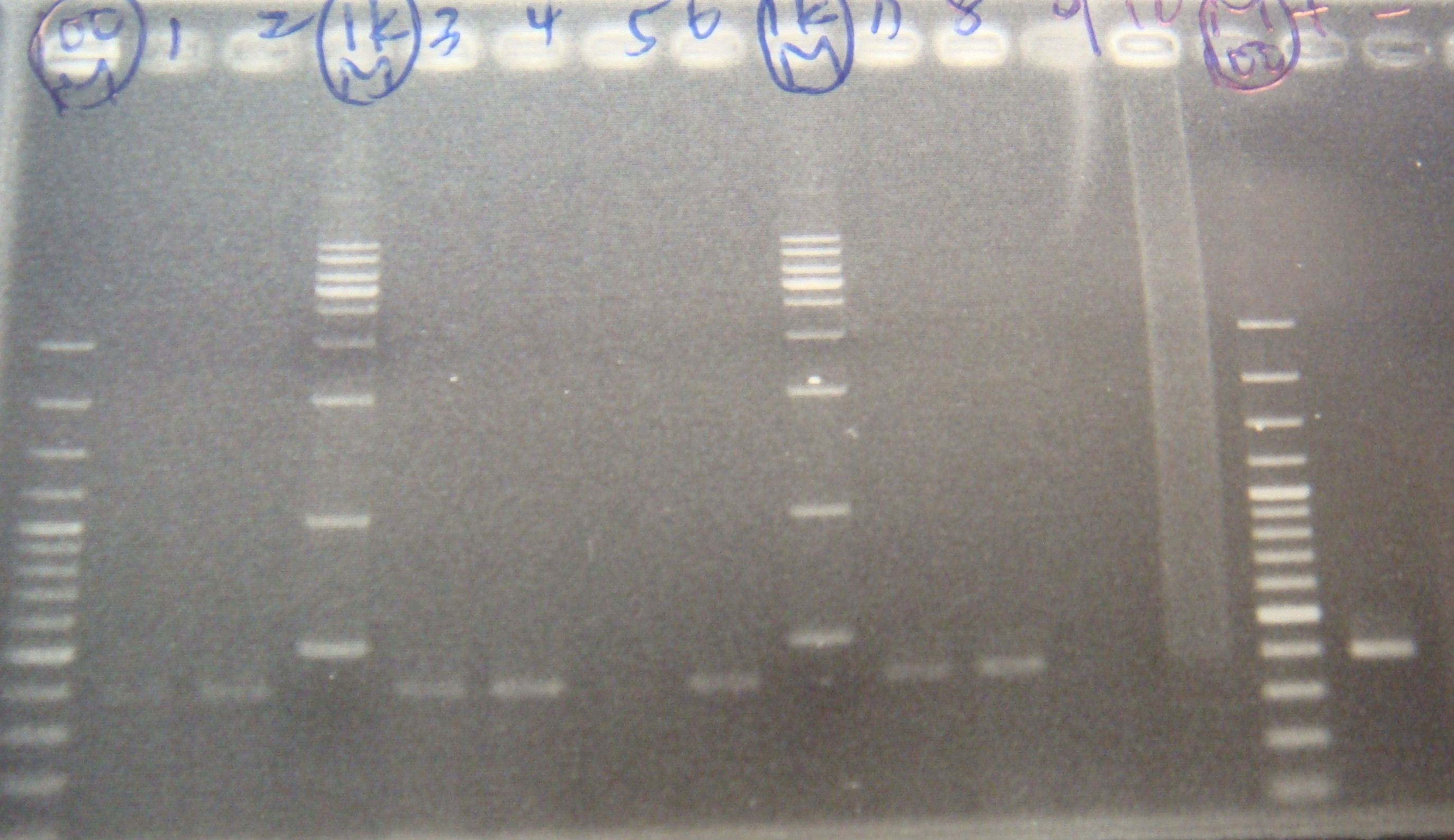
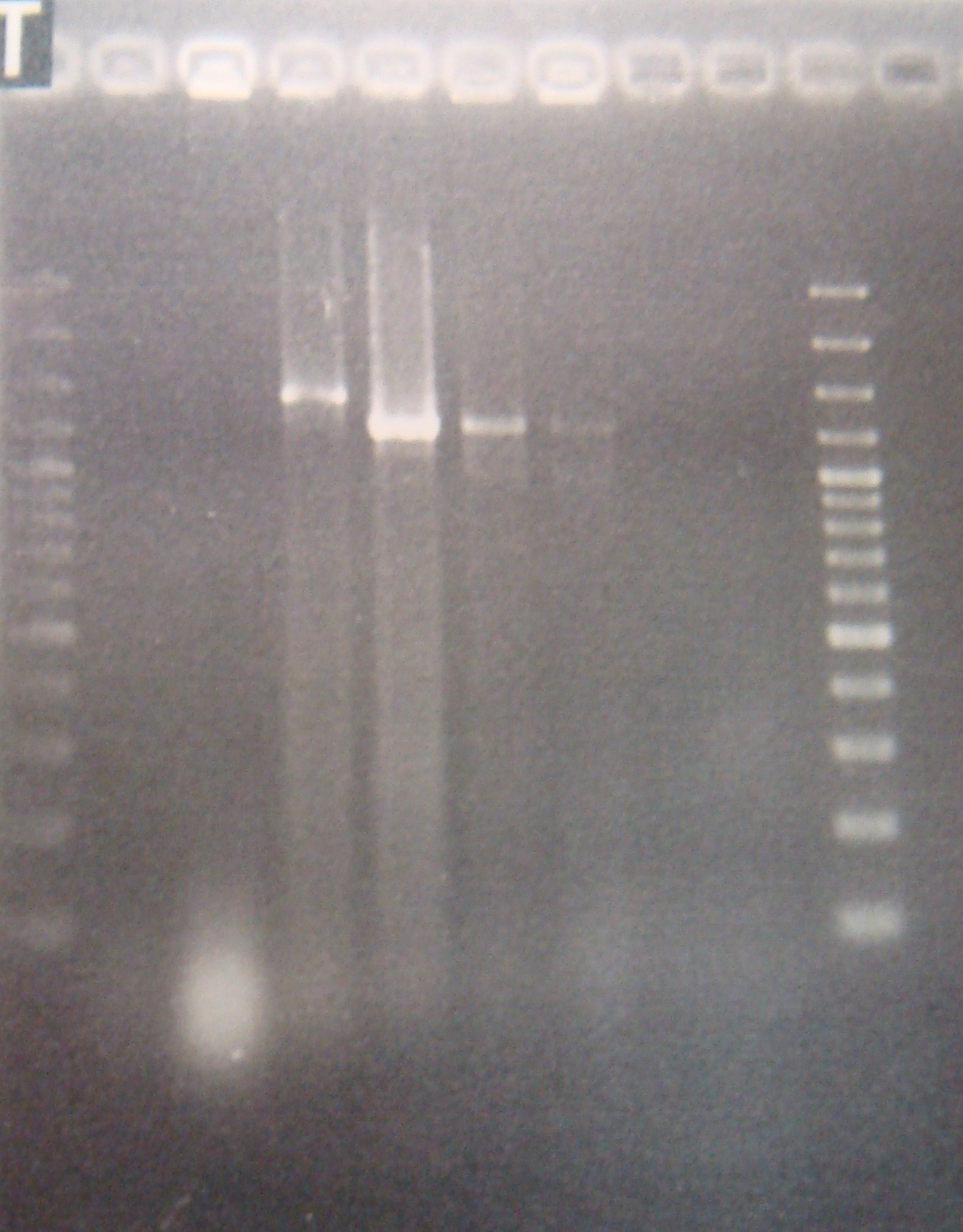
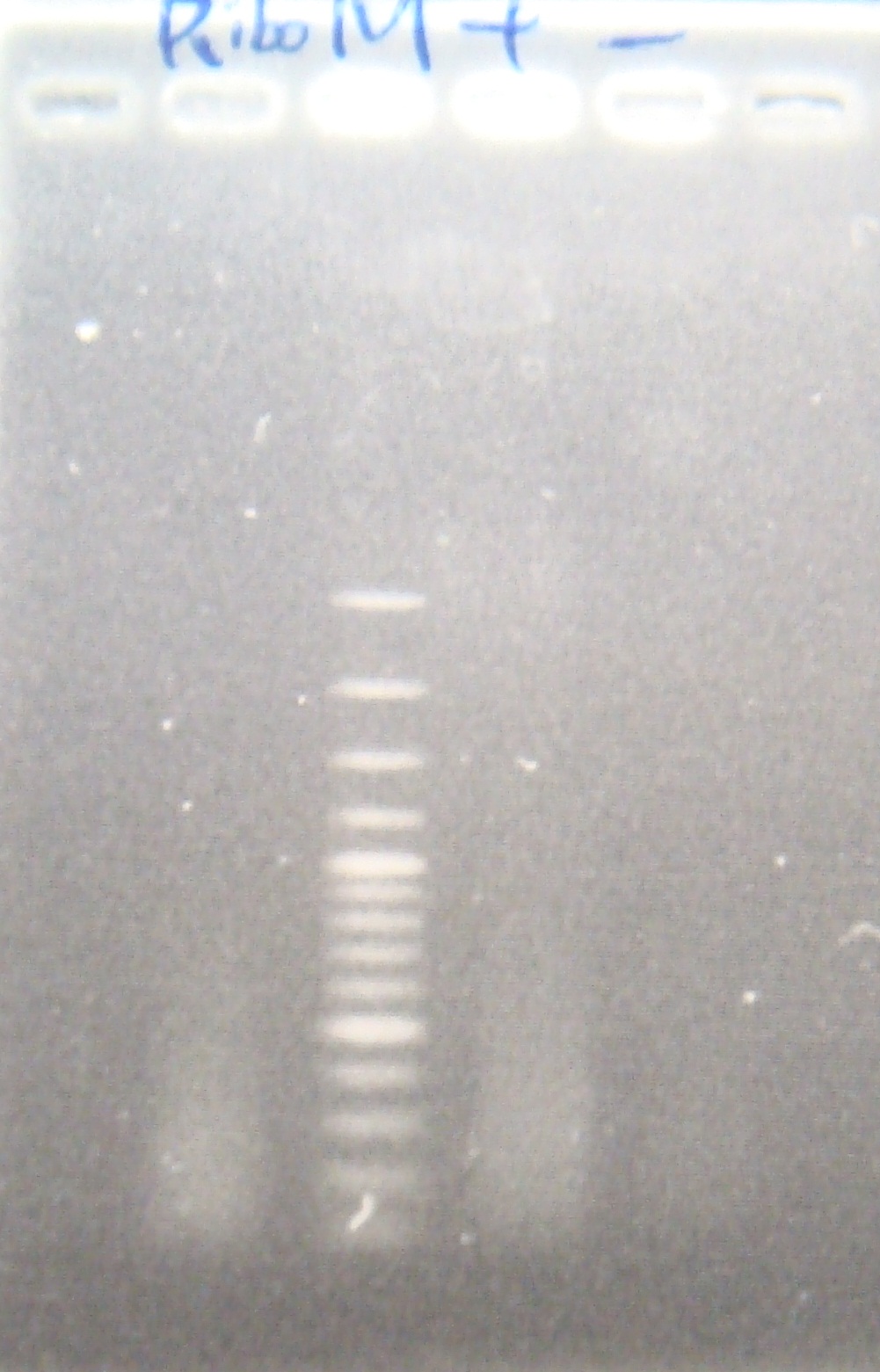
|1: marker 100bp|||n|= | |1: marker 100bp|||n|= | ||
|2: -|-|-|n|= | |2: -|-|-|n|= | ||
| - | |3: riboswitch |56 bp|none| | + | |3: riboswitch |56 bp|none|f|= |
| - | |4: riboswitch |56 bp|none| | + | |4: riboswitch |56 bp|none|f|= |
|5: -|-|-|n|= | |5: -|-|-|n|= | ||
| - | |6: riboswitch |56 bp|none| | + | |6: riboswitch |56 bp|none|f|= |
| - | |7: riboswitch |56 bp|none| | + | |7: riboswitch |56 bp|none|f|= |
|8: -|-|-|n|= | |8: -|-|-|n|= | ||
| - | |9: riboswitch |56 bp|none| | + | |9: riboswitch |56 bp|none|f|= |
| - | |10: riboswitch |56 bp|none| | + | |10: riboswitch |56 bp|none|f|= |
| - | |11: Positive Control|1100 bp|none| | + | |11: Positive Control|1100 bp|none|w|= |
| - | |12: Negative Control||Contamination ~300bp | | + | |12: Negative Control||Contamination ~300bp |w|= |
|}} | |}} | ||
{| border=1 | {| border=1 | ||
| Line 140: | Line 140: | ||
=2010.08.23= | =2010.08.23= | ||
| - | *the upper experiment are failed,so we restart the experiment. | + | *the upper experiment are failed, so we restart the experiment. |
**Run gel 11:00 | **Run gel 11:00 | ||
**After nanodrop, we found that the concentration is too low(only 3.0), so we re-PCR-of-primer again. | **After nanodrop, we found that the concentration is too low(only 3.0), so we re-PCR-of-primer again. | ||
| Line 1,027: | Line 1,027: | ||
{| border="1" | {| border="1" | ||
|- | |- | ||
| - | ! Total: !! 49μl || X1.7 | + | ! Total: !! 49μl || X1.7 || |
|- | |- | ||
| FP || 1μl || 1.7μl || Theophylline Riboswitch Forward Primer | | FP || 1μl || 1.7μl || Theophylline Riboswitch Forward Primer | ||
| Line 1,081: | Line 1,081: | ||
{| border="1" | {| border="1" | ||
|- | |- | ||
| - | ! Total: !! 49μl || X1.9 | + | ! Total: !! 49μl || X1.9 |
|- | |- | ||
| VR+VF|| 2μl || 3.8μl | | VR+VF|| 2μl || 3.8μl | ||
| Line 1,130: | Line 1,130: | ||
{| border="1" | {| border="1" | ||
|- | |- | ||
| - | ! Total: !! 49μl || X2.5 | + | ! Total: !! 49μl || X2.5 |
|- | |- | ||
| VR+VF|| 2μl || 5μl | | VR+VF|| 2μl || 5μl | ||
| Line 1,138: | Line 1,138: | ||
| 10XBuff. || 5μl || 12.5μl | | 10XBuff. || 5μl || 12.5μl | ||
|- | |- | ||
| - | | tag || 0.25μl || 1. | + | | tag || 0.25μl || 1.25μl |
|- | |- | ||
| ddH2O || 39.75μl || 99.375μl | | ddH2O || 39.75μl || 99.375μl | ||
| Line 1,197: | Line 1,197: | ||
{| border="1" | {| border="1" | ||
|- | |- | ||
| - | ! Total: !! 49μl || X1.6 | + | ! Total: !! 49μl || X1.6 |
|- | |- | ||
| VR+VF|| 2μl || 3.2μl | | VR+VF|| 2μl || 3.2μl | ||
| Line 1,205: | Line 1,205: | ||
| 10XBuff. || 5μl || 8μl | | 10XBuff. || 5μl || 8μl | ||
|- | |- | ||
| - | | tag || 0.25μl || 0. | + | | tag || 0.25μl || 0.4μl |
|- | |- | ||
| ddH2O || 39.75μl || 63.6μl | | ddH2O || 39.75μl || 63.6μl | ||
| Line 1,247: | Line 1,247: | ||
{| border="1" | {| border="1" | ||
|- | |- | ||
| - | ! Total: !! 49μl || X1 | + | ! Total: !! 49μl || X1.9 |
|- | |- | ||
| VR+VF|| 2μl || 3.8μl | | VR+VF|| 2μl || 3.8μl | ||
| Line 1,255: | Line 1,255: | ||
| 10XBuff. || 5μl || 9.5μl | | 10XBuff. || 5μl || 9.5μl | ||
|- | |- | ||
| - | | tag || 0.25μl || 0. | + | | tag || 0.25μl || 0.475μl |
|- | |- | ||
| ddH2O || 39.75μl || 75.525μl | | ddH2O || 39.75μl || 75.525μl | ||
| Line 1,301: | Line 1,301: | ||
{| border="1" | {| border="1" | ||
|- | |- | ||
| - | + | | Total: || 49μl || X2.5 | |
|- | |- | ||
| VR+VF|| 2μl || 5μl | | VR+VF|| 2μl || 5μl | ||
| Line 1,309: | Line 1,309: | ||
| 10XBuff. || 5μl || 12.5μl | | 10XBuff. || 5μl || 12.5μl | ||
|- | |- | ||
| - | | tag || 0.25μl || 0. | + | | tag || 0.25μl || 0.625μl |
|- | |- | ||
| ddH2O || 39.75μl || 99.375μl | | ddH2O || 39.75μl || 99.375μl | ||
| Line 1,361: | Line 1,361: | ||
{| border="1" | {| border="1" | ||
|- | |- | ||
| - | ! Total: !! 49μl || | + | ! Total: !! 49μl || X3.8 |
|- | |- | ||
| VR+VF|| 2μl || μl | | VR+VF|| 2μl || μl | ||
| Line 1,408: | Line 1,408: | ||
{| border="1" | {| border="1" | ||
|- | |- | ||
| - | ! Total: !! 49μl || X3.8 | + | ! Total: !! 49μl || X3.8 |
|- | |- | ||
| VR+VF|| 2μl || 7.6μl | | VR+VF|| 2μl || 7.6μl | ||
| Line 1,450: | Line 1,450: | ||
{| border="1" | {| border="1" | ||
|- | |- | ||
| - | ! Total: !! 49μl || X3.8 | + | ! Total: !! 49μl || X3.8 |
|- | |- | ||
| VR+VF|| 2μl || 7.6μl | | VR+VF|| 2μl || 7.6μl | ||
| Line 1,496: | Line 1,496: | ||
{| border="1" | {| border="1" | ||
|- | |- | ||
| - | ! Total: !! 49μl || X3.2 | + | ! Total: !! 49μl || X3.2 |
|- | |- | ||
| VR+VF|| 2μl || 6.4μl | | VR+VF|| 2μl || 6.4μl | ||
| Line 1,555: | Line 1,555: | ||
{| border="1" | {| border="1" | ||
|- | |- | ||
| - | ! Total: !! 49μl || X1.7 | + | ! Total: !! 49μl || X1.7 |
|- | |- | ||
| VR+VF|| 2μl || 3.4μl | | VR+VF|| 2μl || 3.4μl | ||
| Line 1,612: | Line 1,612: | ||
{| border="1" | {| border="1" | ||
|- | |- | ||
| - | ! Total: !! 49μl || X2.5 | + | ! Total: !! 49μl || X2.5 |
|- | |- | ||
| VR+VF|| 2μl || 5μl | | VR+VF|| 2μl || 5μl | ||
| Line 1,656: | Line 1,656: | ||
{| border="1" | {| border="1" | ||
|- | |- | ||
| - | ! Total: !! 49μl || X2.5 | + | ! Total: !! 49μl || X2.5 |
|- | |- | ||
| VR+VF|| 2μl || 5μl | | VR+VF|| 2μl || 5μl | ||
| Line 1,664: | Line 1,664: | ||
| 10XBuff. || 5μl || 12.5μl | | 10XBuff. || 5μl || 12.5μl | ||
|- | |- | ||
| - | | tag || 0.25μl || 0. | + | | tag || 0.25μl || 0.625μl |
|- | |- | ||
| ddH2O || 39.75μl || 99.375μl | | ddH2O || 39.75μl || 99.375μl | ||
| Line 1,721: | Line 1,721: | ||
{| border="1" | {| border="1" | ||
|- | |- | ||
| - | ! Total: !! 49μl || X1.3 | + | ! Total: !! 49μl || X1.3 |
|- | |- | ||
| VR+VF|| 2μl || 2.6μl | | VR+VF|| 2μl || 2.6μl | ||
| Line 1,729: | Line 1,729: | ||
| 10XBuff. || 5μl || 6.5μl | | 10XBuff. || 5μl || 6.5μl | ||
|- | |- | ||
| - | | tag || 0.25μl || 0. | + | | tag || 0.25μl || 0.325μl |
|- | |- | ||
| ddH2O || 39.75μl || 51.675μl | | ddH2O || 39.75μl || 51.675μl | ||
| Line 1,805: | Line 1,805: | ||
{| border="1" | {| border="1" | ||
|- | |- | ||
| - | ! Total: !! 49μl || X2.1 | + | ! Total: !! 49μl || X2.1 |
|- | |- | ||
| VR+VF|| 2μl || 4.2μl | | VR+VF|| 2μl || 4.2μl | ||
| Line 1,813: | Line 1,813: | ||
| 10XBuff. || 5μl || 10.5μl | | 10XBuff. || 5μl || 10.5μl | ||
|- | |- | ||
| - | | tag || 0.25μl || 0. | + | | tag || 0.25μl || 0.525μl |
|- | |- | ||
| ddH2O || 39.75μl || 83.475μl | | ddH2O || 39.75μl || 83.475μl | ||
| Line 1,909: | Line 1,909: | ||
{| border="1" | {| border="1" | ||
|- | |- | ||
| - | ! Total: !! 49μl || X2.5 | + | ! Total: !! 49μl || X2.5 |
|- | |- | ||
| VR+VF|| 2μl || 5μl | | VR+VF|| 2μl || 5μl | ||
| Line 1,917: | Line 1,917: | ||
| 10XBuff. || 5μl || 12.5μl | | 10XBuff. || 5μl || 12.5μl | ||
|- | |- | ||
| - | | tag || 0.25μl || 1. | + | | tag || 0.25μl || 1.25μl |
|- | |- | ||
| ddH2O || 39.75μl || 99.375μl | | ddH2O || 39.75μl || 99.375μl | ||
| Line 1,953: | Line 1,953: | ||
*Transform | *Transform | ||
------------------------------- | ------------------------------- | ||
| - | *Theophylline solution | + | *Preparing 0.1 M Theophylline solution in DMSO. |
*Assay | *Assay | ||
{| border=1 | {| border=1 | ||
| Line 2,026: | Line 2,026: | ||
{| border="1" | {| border="1" | ||
|- | |- | ||
| - | ! Total: !! 49μl || X0.9 | + | ! Total: !! 49μl || X0.9 |
|- | |- | ||
| VR+VF|| 2μl || 1.8μl | | VR+VF|| 2μl || 1.8μl | ||
| Line 2,034: | Line 2,034: | ||
| 10XBuff. || 5μl || 4.5μl | | 10XBuff. || 5μl || 4.5μl | ||
|- | |- | ||
| - | | tag || 0.25μl || 0. | + | | tag || 0.25μl || 0.225μl |
|- | |- | ||
| ddH2O || 39.75μl || 35.775μl | | ddH2O || 39.75μl || 35.775μl | ||
| Line 2,069: | Line 2,069: | ||
{| border="1" | {| border="1" | ||
|- | |- | ||
| - | ! Total: !! 49μl || X1.6 | + | ! Total: !! 49μl || X1.6 |
|- | |- | ||
| VR+VF|| 2μl || 3.2μl | | VR+VF|| 2μl || 3.2μl | ||
| Line 2,077: | Line 2,077: | ||
| 10XBuff. || 5μl || 8μl | | 10XBuff. || 5μl || 8μl | ||
|- | |- | ||
| - | | tag || 0.25μl || 0. | + | | tag || 0.25μl || 0.8μl |
|- | |- | ||
| ddH2O || 39.75μl || 63μl | | ddH2O || 39.75μl || 63μl | ||
| Line 2,154: | Line 2,154: | ||
{| border="1" | {| border="1" | ||
|- | |- | ||
| - | ! Total: !! 49μl || X2.4 | + | ! Total: !! 49μl || X2.4 |
|- | |- | ||
| VR+VF|| 2μl || 4.8μl | | VR+VF|| 2μl || 4.8μl | ||
| Line 2,162: | Line 2,162: | ||
| 10XBuff. || 5μl || 12μl | | 10XBuff. || 5μl || 12μl | ||
|- | |- | ||
| - | | tag || 0.25μl || 0. | + | | tag || 0.25μl || 0.6μl |
|- | |- | ||
| ddH2O || 39.75μl || 95.4μl | | ddH2O || 39.75μl || 95.4μl | ||
Latest revision as of 23:56, 27 October 2010
| Home | Project Overview | Speedy reporter | Speedy switch | Speedy protein degrader | Experiments and Parts | Applications | F.A.Q | About Us |
Parts
- Ribo = Theophylline riboswitch([http://partsregistry.org/Part:BBa_K411001 BBa_K411001])
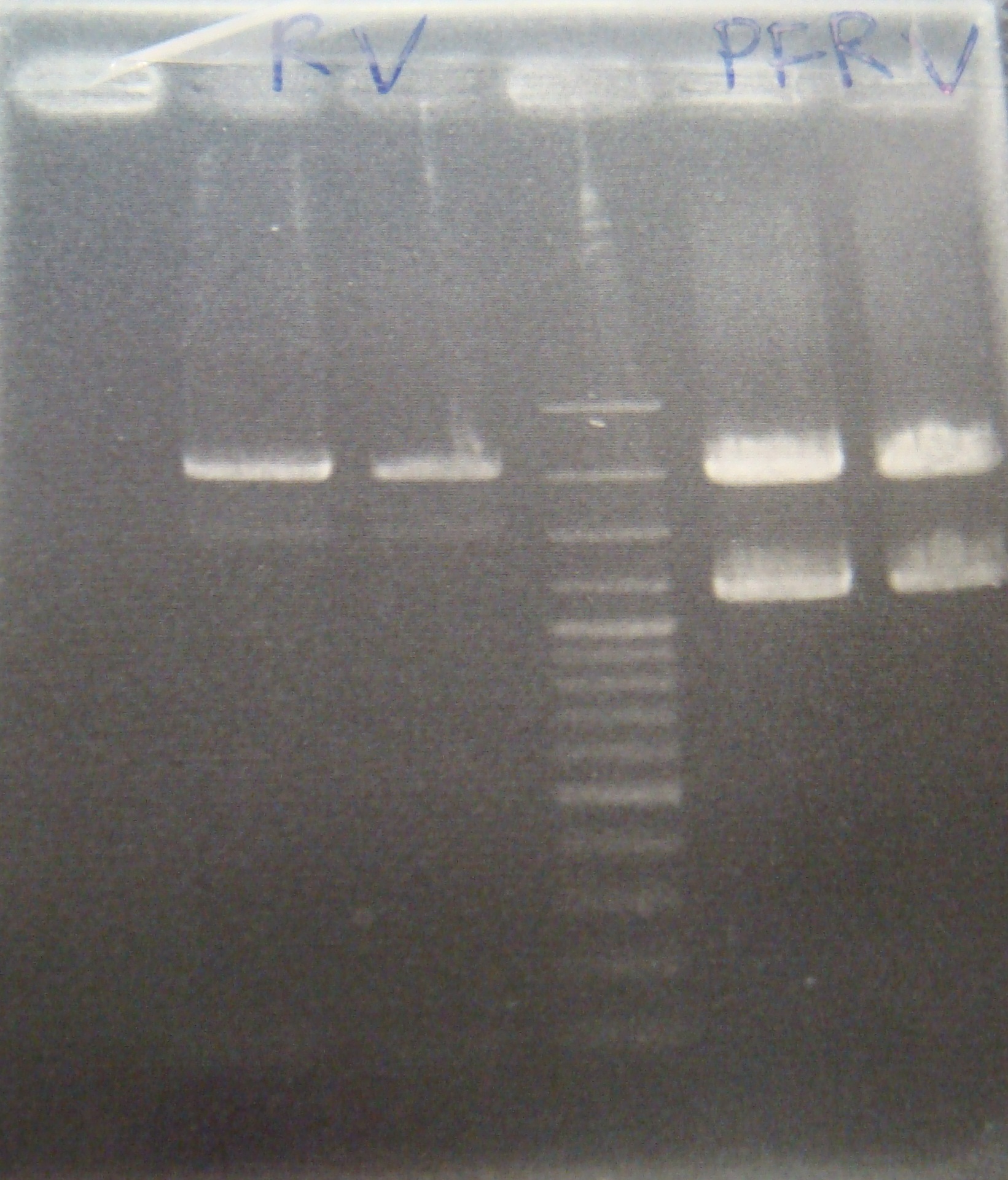
- RV = Theophylline riboswitch([http://partsregistry.org/Part:BBa_K411001 BBa_K411001]) + pSB1A2
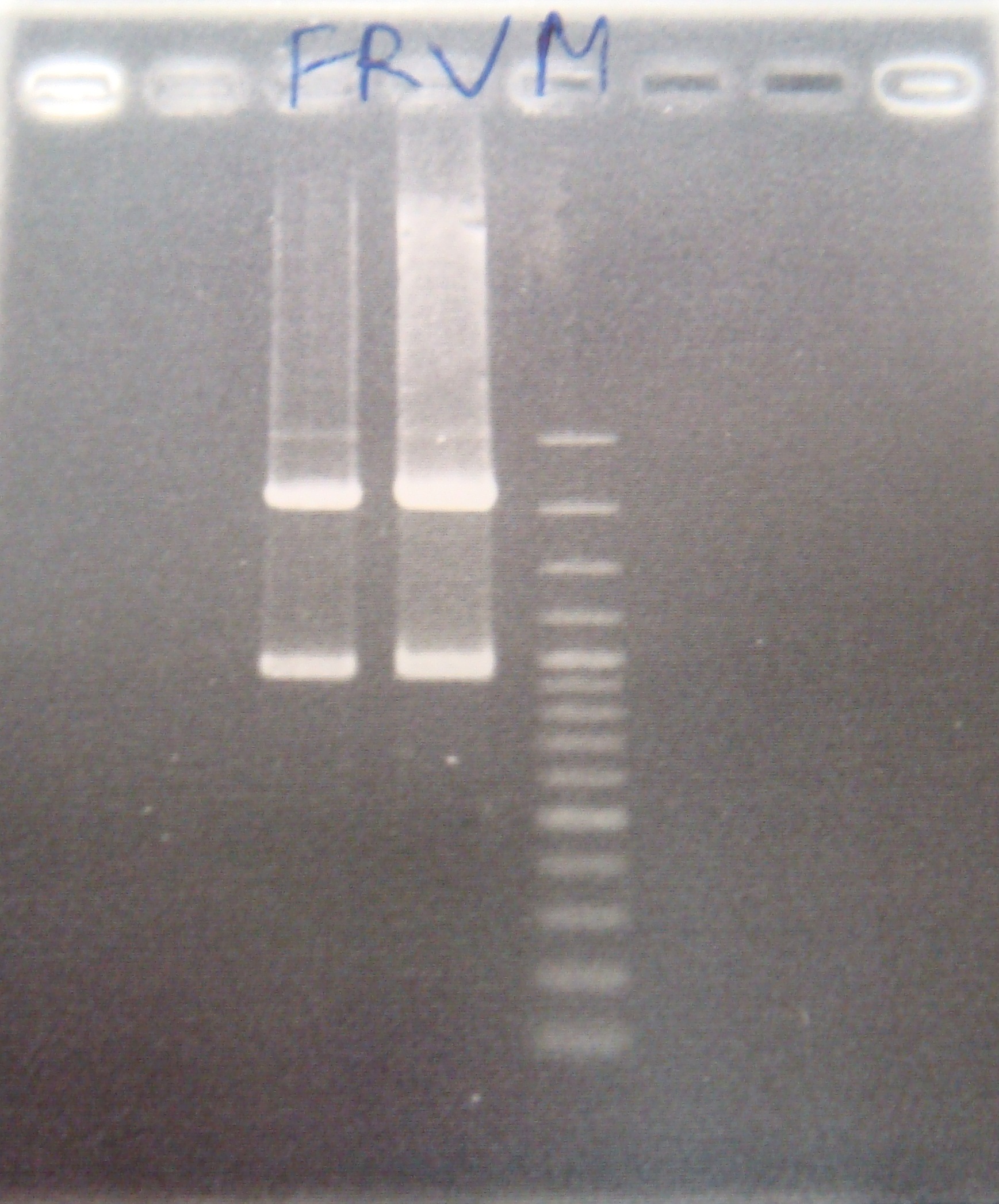
- FRV = Theophylline riboswitch + GFP([http://partsregistry.org/Part:BBa_J04630 BBa_J04630]) + pSB1A2
- PFRV = Theophylline riboswitch + GFP([http://partsregistry.org/Part:BBa_J04630 BBa_J04630]) + pLac([http://partsregistry.org/Part:BBa_R0010 BBa_R0010]) + pSB1A2
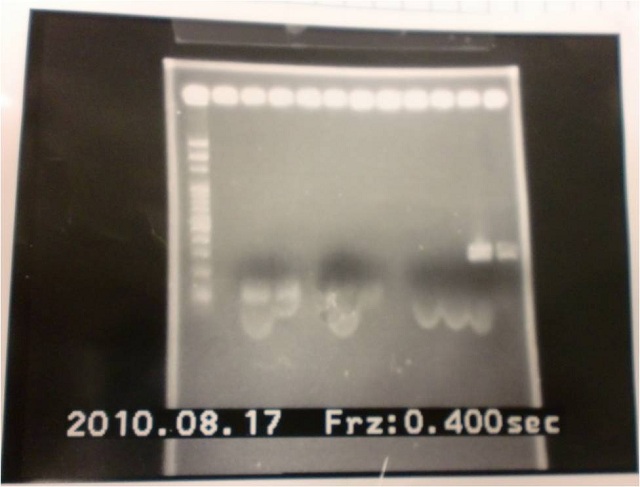
2010.08.17
- PCR of primer&Digestion&Ligation
- Plasmid extraction
- Digestion
- PCR purification(centrifuge)
- Nanodrop
- Ligation
- Transform 20:00
2010.08.18
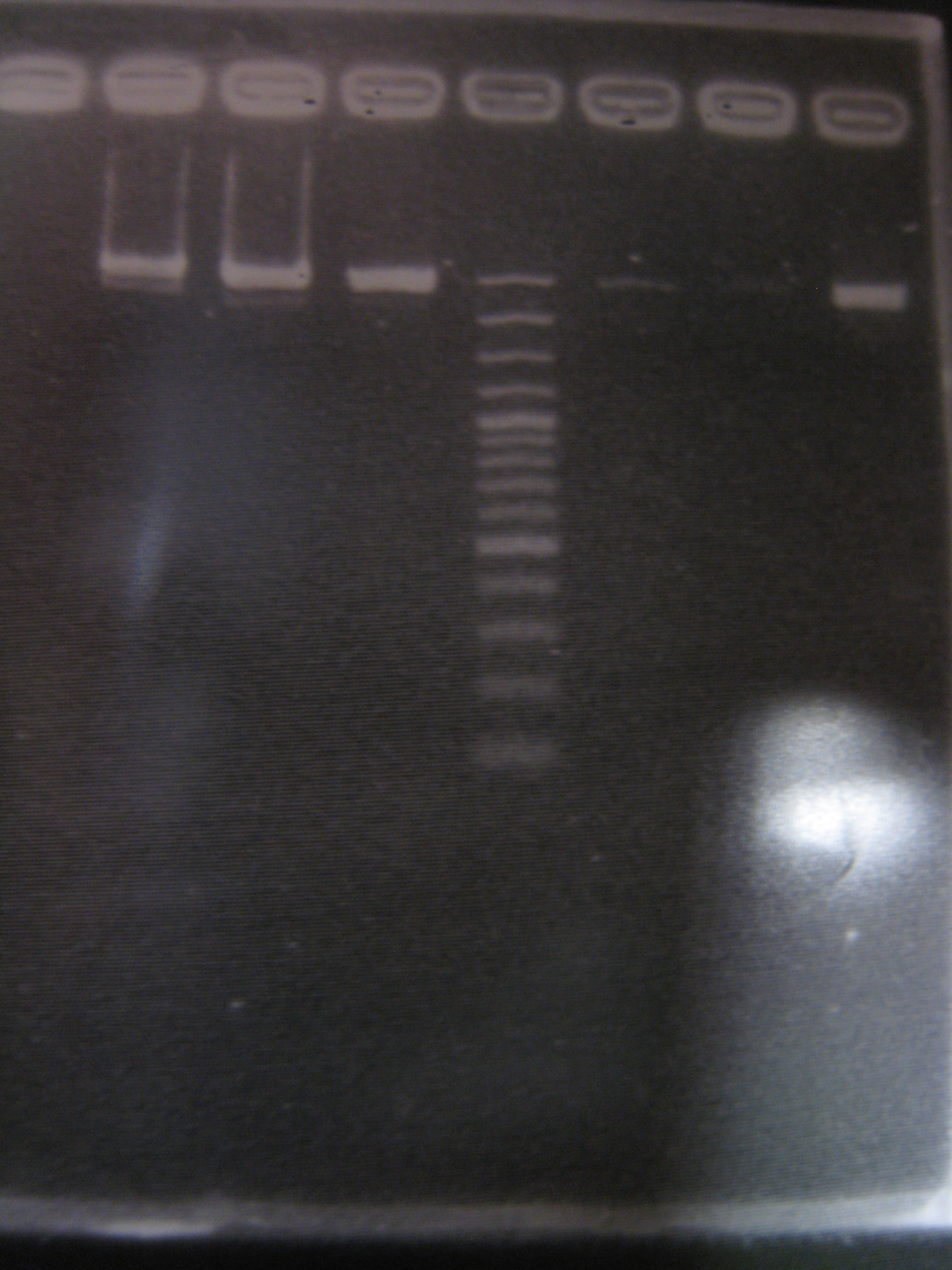
- PCR mix
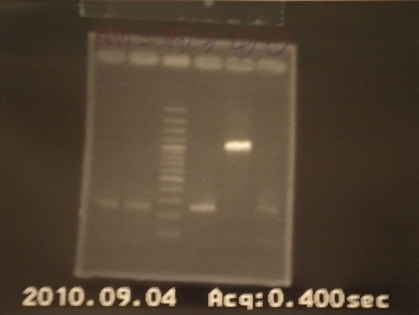
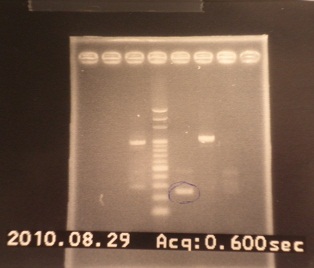
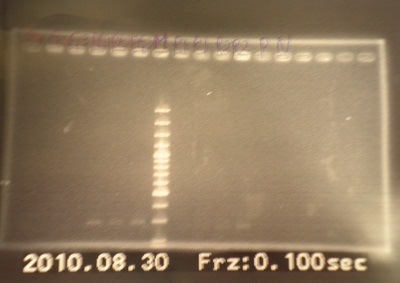
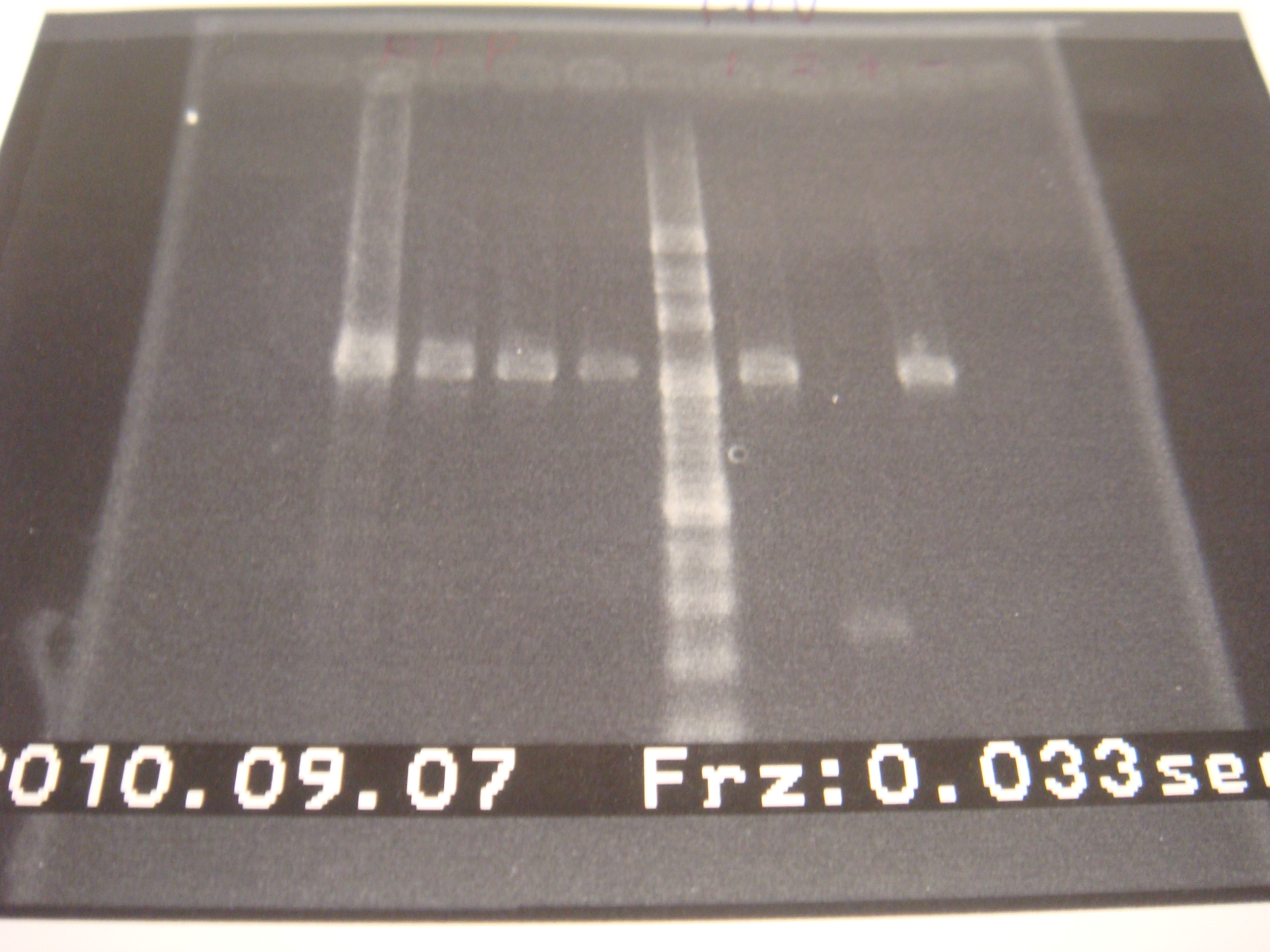
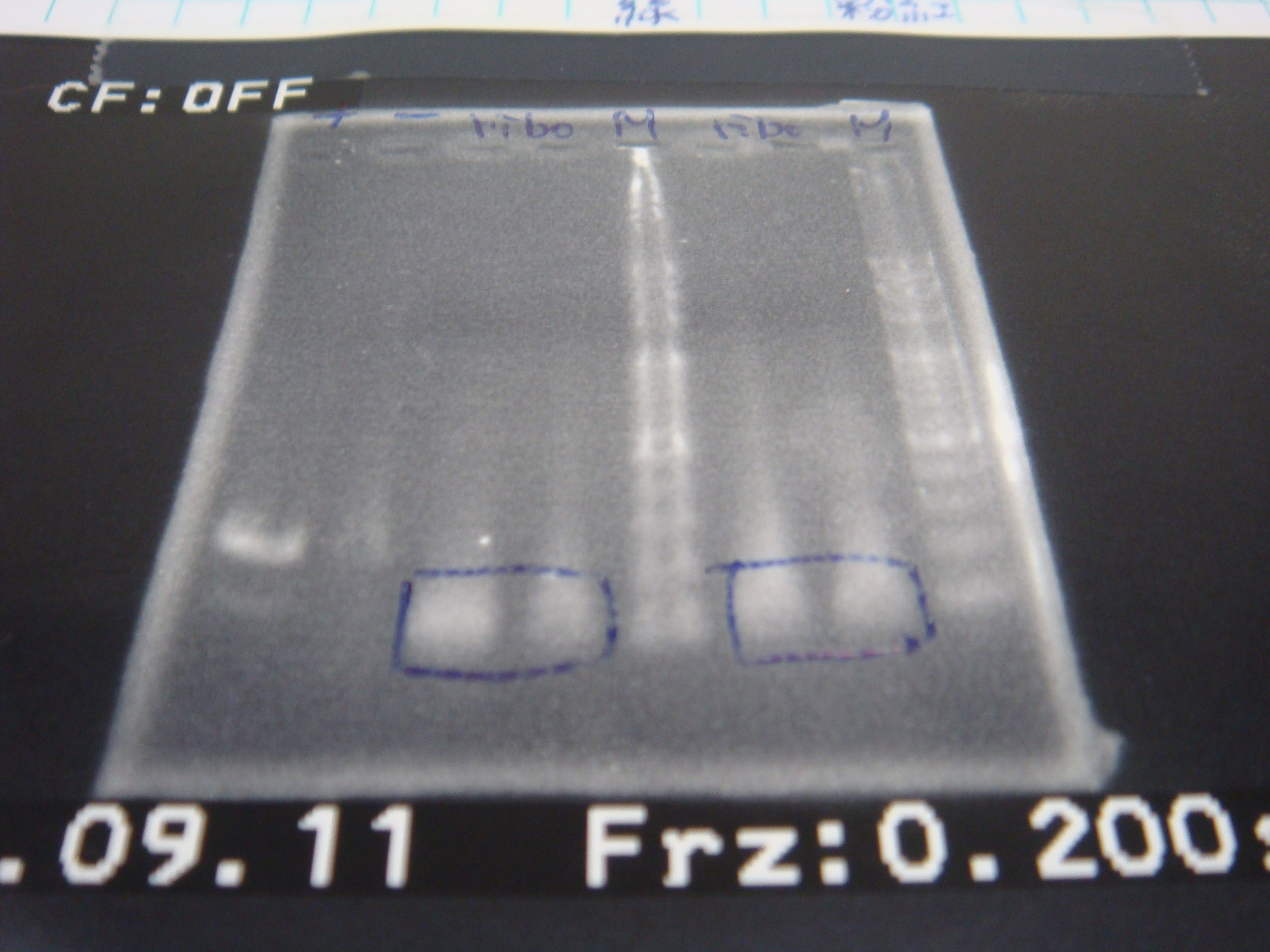
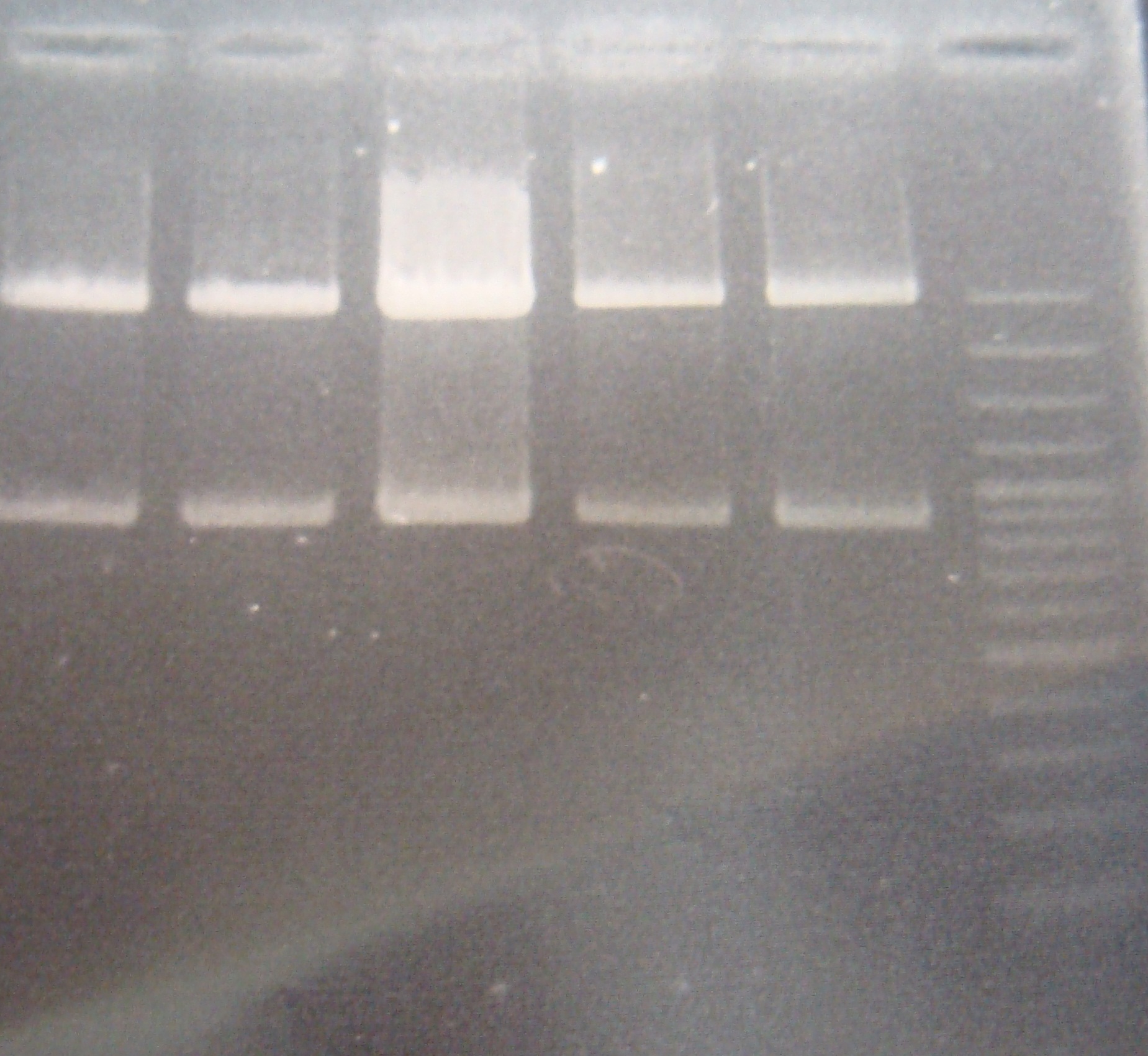
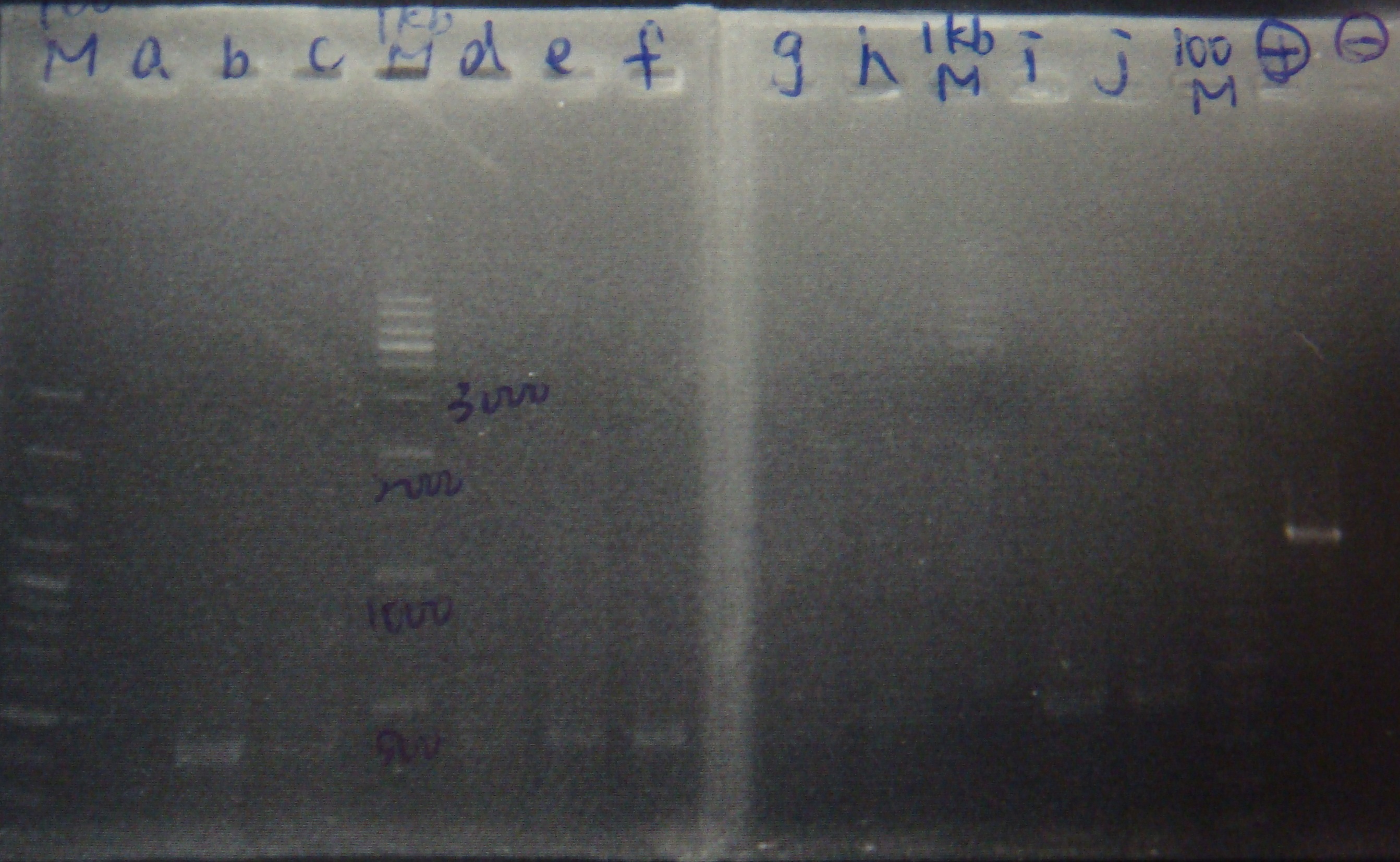
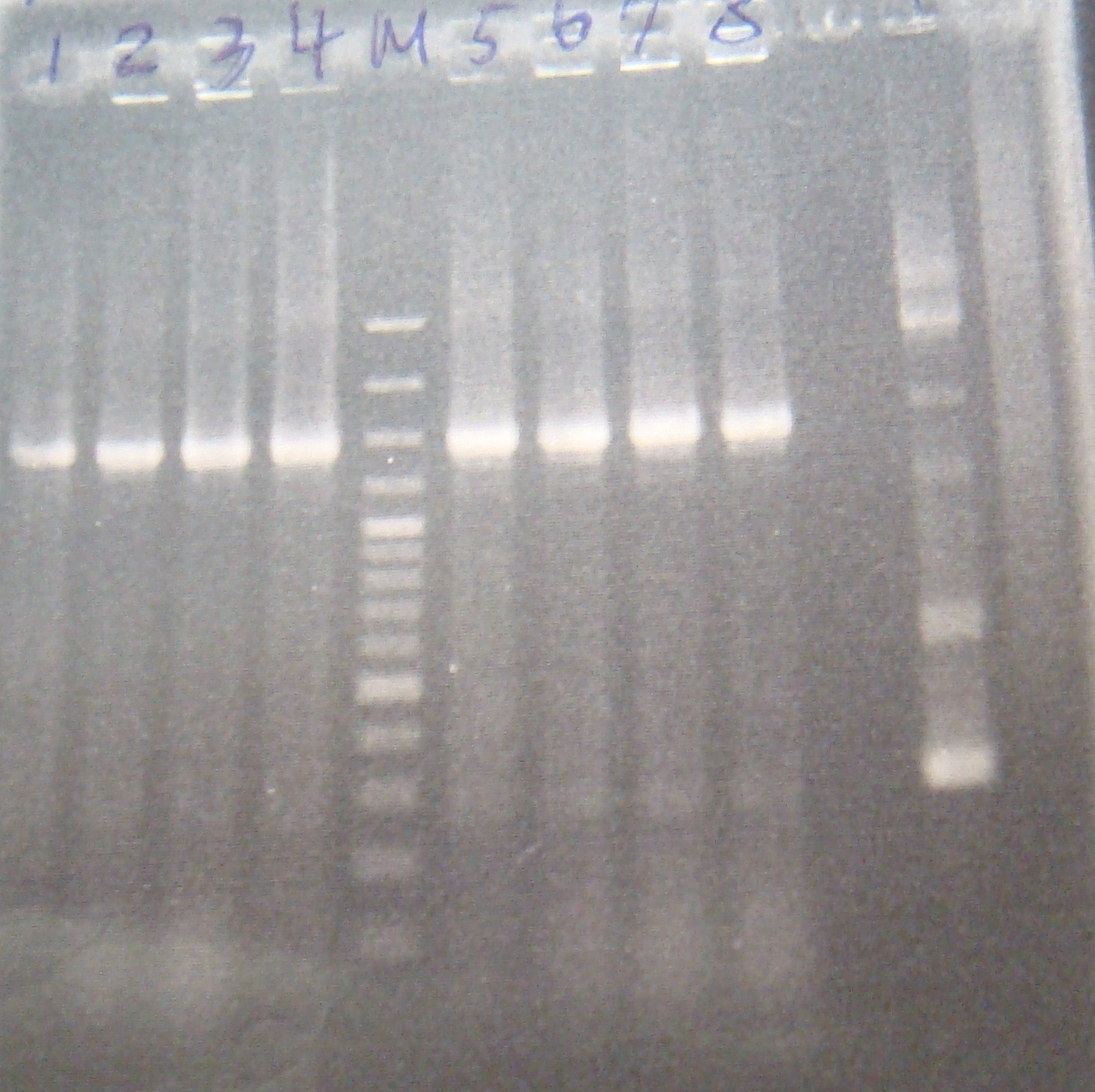
- Run gel(2%argarose 100v)
 |
|
|---|
(negative contaminated and appeared three different bands)
- 3-in-1 11:30
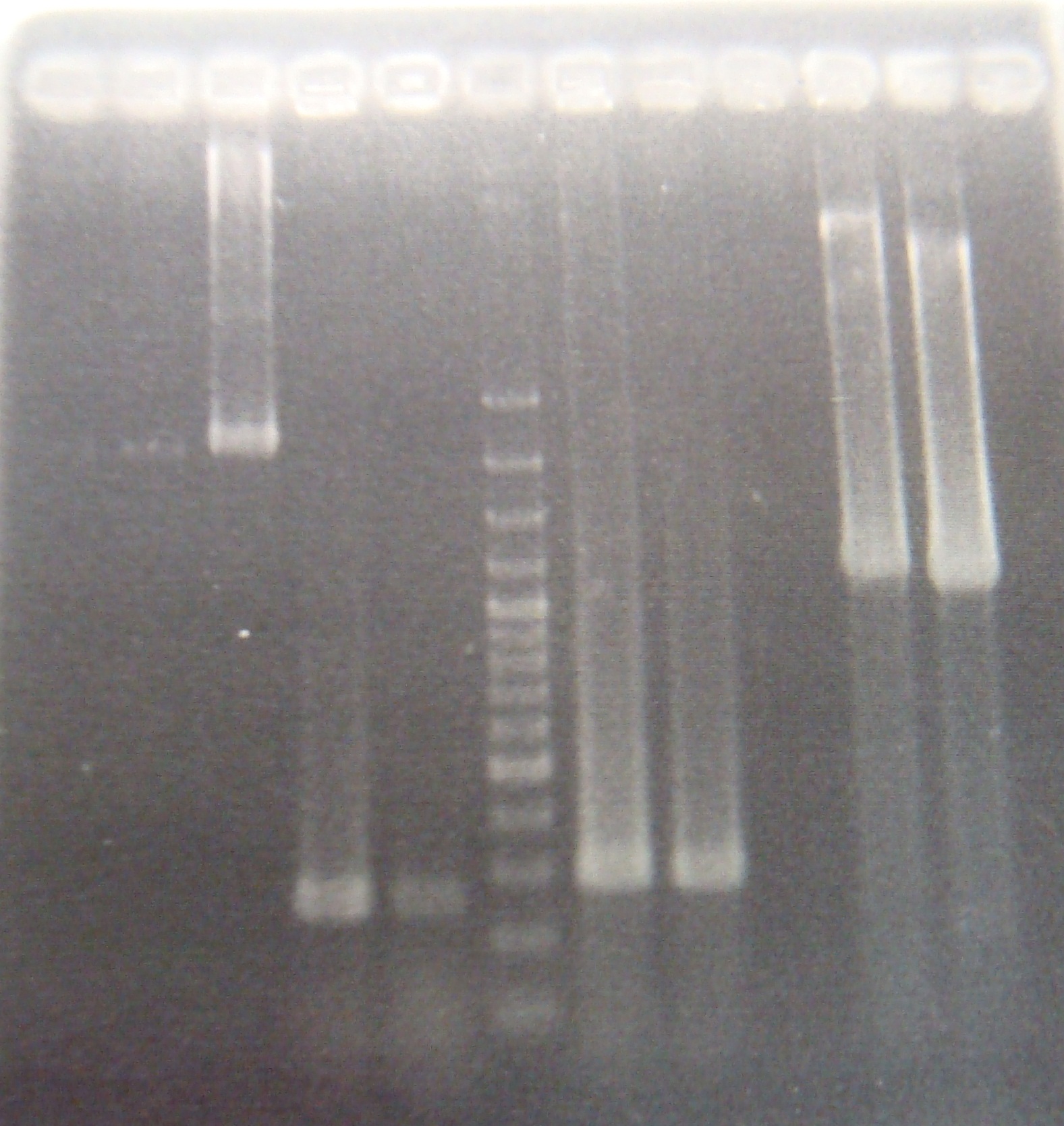
- Run PCR 12:00
- put Liquid culture&plate at 17:00
2010.08.19
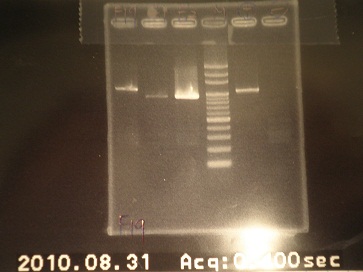
- Because there are some mistake resulting from gel running, we decided to digest again.
- Digest 10:30
- Transformation 12:45
- At the same time, we continued plasmid extraction of the result one of yesterday.
- plasmid extraction 10:30
- Digest 11:30
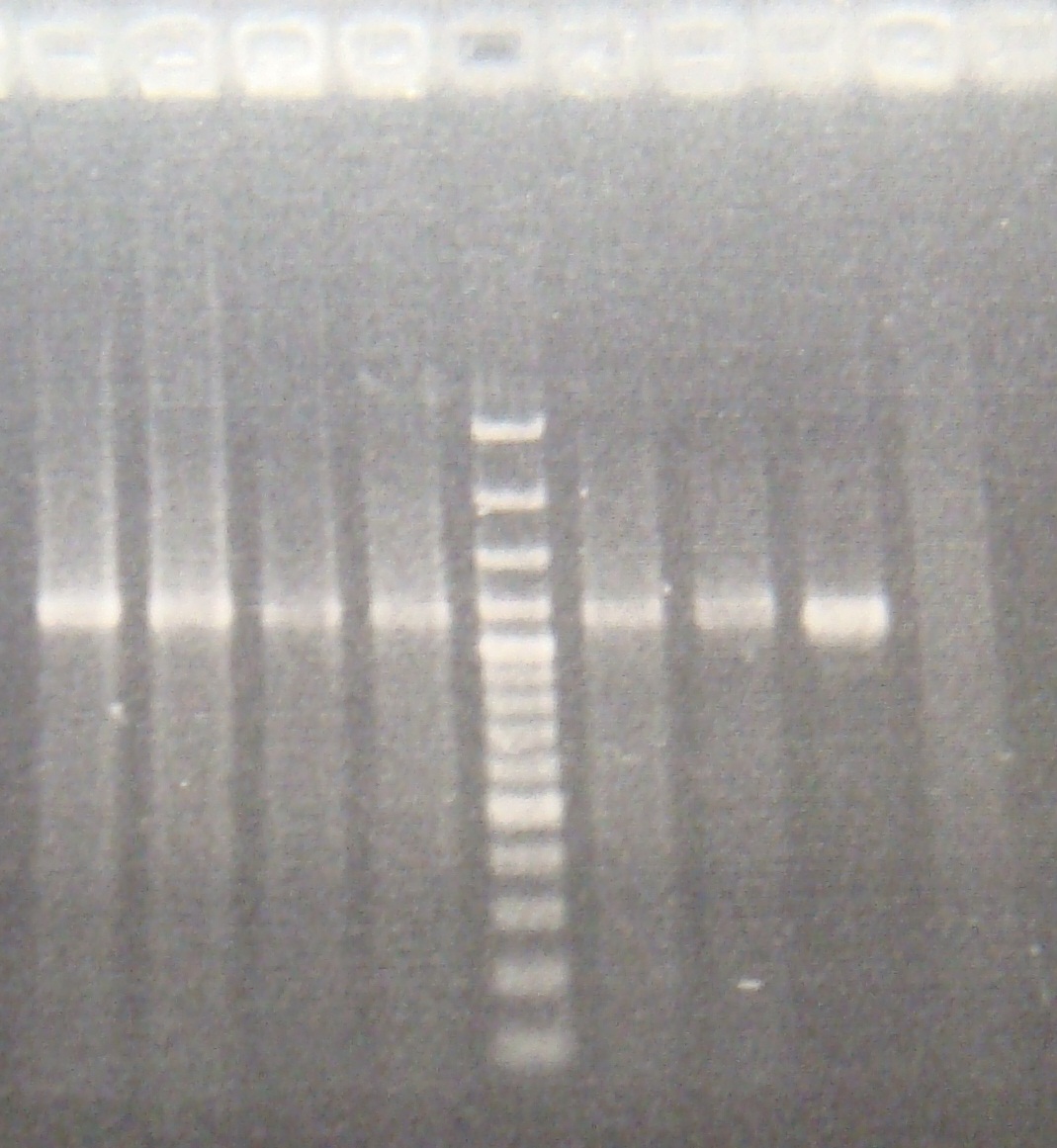
- run gel 13:00
- Ligation 14:00
- Transformation 14:30
2010.08.20
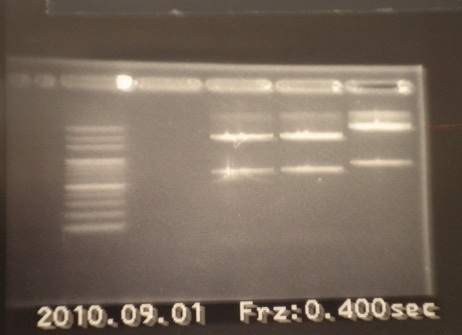
- We decide to re-3-in-1 again.
- PCR mix 10:30
- 3 in 1 11:30
- Run PCR 01:00~02:30
- Run gel 15:00(100v 20 mins)
2010.08.23
2010.08.24
|
 "
"