Team:HokkaidoU Japan/Notebook/September29
From 2010.igem.org
(Difference between revisions)
| (6 intermediate revisions not shown) | |||
| Line 3: | Line 3: | ||
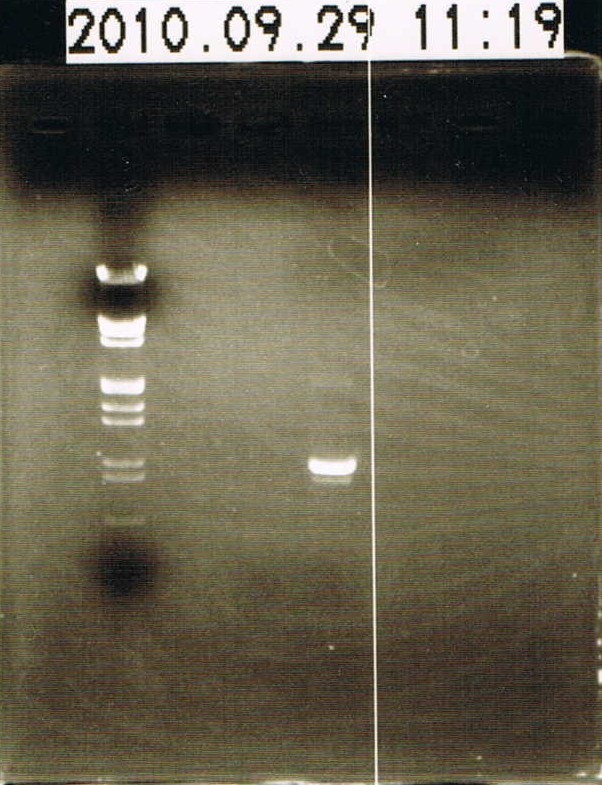
= Electrophoresis of Yesterday Samples = | = Electrophoresis of Yesterday Samples = | ||
| - | |||
*electrophoresed 6 uL Marker λ/HindIII EcoRI and 1 uL of two yesterday samples.T3SS signal wasn't observed. | *electrophoresed 6 uL Marker λ/HindIII EcoRI and 1 uL of two yesterday samples.T3SS signal wasn't observed. | ||
| - | + | [[Image:HokkaidoU Japan 20100929a.jpg|200px|center|thumb|Electrophoresis of concentrated GFP-double terminator(T3SS signal wasn't seen)]] | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
= Colony PCR of Arabinose Promoter + RBS + GFP + double terminator = | = Colony PCR of Arabinose Promoter + RBS + GFP + double terminator = | ||
| Line 83: | Line 53: | ||
*erectrophoresed the samples.Marker is 10 uL λ/HindIII EcoRI. | *erectrophoresed the samples.Marker is 10 uL λ/HindIII EcoRI. | ||
| - | [[Image:HokkaidoU Japan 20100929b.jpg|200px| | + | [[Image:HokkaidoU Japan 20100929b.jpg|200px|center|thumb|Electrophoresis of colony PCR sample]] |
| + | = Cultivation of colony = | ||
| + | *cultivated the colonies which were used for PCR.Each Samples were cultured in LB contained Ampicillin and 20% Arabinose or contained only Ampicillin. | ||
| + | = PCR of T3SS signal again = | ||
| + | *We thought colony PCR can't use KOD plus neo.We did a PCR with KOD plus neo again,and also did with Quick Taq. | ||
| + | PCR mix | ||
| + | <div style="float:left;"> | ||
| + | {|style="text-align: center" class="protocol" | ||
| + | !Reagent | ||
| + | !Amount | ||
| + | |- | ||
| + | |colony solution | ||
| + | |10 uL | ||
| + | |- | ||
| + | |DW | ||
| + | |23 uL | ||
| + | |- | ||
| + | |10x PCR Buffer | ||
| + | |5 uL | ||
| + | |- | ||
| + | |5 mM 4dNTPs | ||
| + | |5 uL | ||
| + | |- | ||
| + | |25 mM MgSO4 | ||
| + | |3 uL | ||
| + | |- | ||
| + | |EX-RBS Primer | ||
| + | |1.5 uL | ||
| + | |- | ||
| + | |SlrP3 Primer | ||
| + | |1.5 uL | ||
| + | |- | ||
| + | |KOD plus neo | ||
| + | |1 uL | ||
| + | |- | ||
| + | |style="border-top:1px solid #996"|'''Total''' | ||
| + | |style="border-top:1px solid #996"|'''50 uL''' | ||
| + | |} | ||
| + | </div> | ||
| + | <div style="float:left;"> | ||
| + | {|style="text-align: center" class="protocol" | ||
| + | !Reagent | ||
| + | !Amount | ||
| + | |- | ||
| + | |Quick Taq | ||
| + | |7 uL | ||
| + | |- | ||
| + | |EX-F | ||
| + | |1.5 uL | ||
| + | |- | ||
| + | |PS-R | ||
| + | |1.5 uL | ||
| + | |- | ||
| + | |colony solution | ||
| + | |10 uL | ||
| + | |- | ||
| + | |style="border-top:1px solid #996"|'''Total''' | ||
| + | |style="border-top:1px solid #996"|'''20 uL''' | ||
| + | |} | ||
| + | </div> | ||
| Line 113: | Line 142: | ||
| - | |||
| - | * | + | |
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | *PCRed according to the table below.98C and 68C for 45 cycles in PCR of KOD. 94C 30 sec and 68C for 40 cycle in PCR of Quick Taq. | ||
| + | |||
| + | <br> | ||
| + | <div style="float:left;"> | ||
| + | {|style="text-align: center" class="protocol" | ||
| + | !temp | ||
| + | !time | ||
| + | |- | ||
| + | |94C | ||
| + | |2 min | ||
| + | |- | ||
| + | |98C | ||
| + | |10 sec | ||
| + | |- | ||
| + | |68C | ||
| + | |60 sec | ||
| + | |- | ||
| + | |4C | ||
| + | |hold | ||
| + | |- | ||
| + | |} | ||
| + | </div> | ||
| + | <div style="float:left;"> | ||
| + | {|style="text-align: center" class="protocol" | ||
| + | !temp | ||
| + | !time | ||
| + | |- | ||
| + | |94C | ||
| + | |2 min | ||
| + | |- | ||
| + | |94C | ||
| + | |30 sec | ||
| + | |- | ||
| + | |68C | ||
| + | |90 sec | ||
| + | |- | ||
| + | |4C | ||
| + | |hold | ||
| + | |- | ||
| + | |} | ||
| + | </div> | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | *Electrophoresed the samples and pSB1C3 and pSB1T3 which were amplified before.A band of T3SS signal amplified by Quick Taq was observed, but by KOD plus neo wasn't done.Estimated a concentration of T3SS signal and plasmid. | ||
Latest revision as of 17:28, 27 October 2010
Electrophoresis of Yesterday Samples
- electrophoresed 6 uL Marker λ/HindIII EcoRI and 1 uL of two yesterday samples.T3SS signal wasn't observed.
Colony PCR of Arabinose Promoter + RBS + GFP + double terminator
- PCRed 5 colony.
PCR mix
| Reagent | Amount |
|---|---|
| Quick Taq | 25 uL |
| EX-F | 0.5 uL |
| PS-R | 0.5 uL |
| Total | 26 uL |
- PCRed according to the table below.98C and 68C for 35 cycles.
| temp | time |
|---|---|
| 94C | 2 min |
| 94C | 30 sec |
| 68C | 90 sec |
| 4C | hold |
- erectrophoresed the samples.Marker is 10 uL λ/HindIII EcoRI.
Cultivation of colony
- cultivated the colonies which were used for PCR.Each Samples were cultured in LB contained Ampicillin and 20% Arabinose or contained only Ampicillin.
PCR of T3SS signal again
- We thought colony PCR can't use KOD plus neo.We did a PCR with KOD plus neo again,and also did with Quick Taq.
PCR mix
| Reagent | Amount |
|---|---|
| colony solution | 10 uL |
| DW | 23 uL |
| 10x PCR Buffer | 5 uL |
| 5 mM 4dNTPs | 5 uL |
| 25 mM MgSO4 | 3 uL |
| EX-RBS Primer | 1.5 uL |
| SlrP3 Primer | 1.5 uL |
| KOD plus neo | 1 uL |
| Total | 50 uL |
| Reagent | Amount |
|---|---|
| Quick Taq | 7 uL |
| EX-F | 1.5 uL |
| PS-R | 1.5 uL |
| colony solution | 10 uL |
| Total | 20 uL |
- PCRed according to the table below.98C and 68C for 45 cycles in PCR of KOD. 94C 30 sec and 68C for 40 cycle in PCR of Quick Taq.
| temp | time |
|---|---|
| 94C | 2 min |
| 98C | 10 sec |
| 68C | 60 sec |
| 4C | hold |
| temp | time |
|---|---|
| 94C | 2 min |
| 94C | 30 sec |
| 68C | 90 sec |
| 4C | hold |
- Electrophoresed the samples and pSB1C3 and pSB1T3 which were amplified before.A band of T3SS signal amplified by Quick Taq was observed, but by KOD plus neo wasn't done.Estimated a concentration of T3SS signal and plasmid.
 "
"