Team:Korea U Seoul/Notebook
From 2010.igem.org
(→[ Digestion ] 2010-10-01 ~ 2010-10-03) |
|||
| (49 intermediate revisions not shown) | |||
| Line 25: | Line 25: | ||
<div id="yong"> | <div id="yong"> | ||
<!--- The Mission, Experiments ---> | <!--- The Mission, Experiments ---> | ||
| - | + | ||
='''Brain storming & Work notes'''= | ='''Brain storming & Work notes'''= | ||
Click on a date to see notes on the meeting & summary of labwork done on that day. | Click on a date to see notes on the meeting & summary of labwork done on that day. | ||
| Line 40: | Line 40: | ||
<br> | <br> | ||
='''Experimental notes'''= | ='''Experimental notes'''= | ||
| - | |||
| - | |||
| - | |||
| - | |||
== [Discussion] 2010-08-02 ~ 2010-08-29 == | == [Discussion] 2010-08-02 ~ 2010-08-29 == | ||
| Line 57: | Line 53: | ||
!Sequence ( 5’ → 3’ ) | !Sequence ( 5’ → 3’ ) | ||
|- | |- | ||
| - | | | + | |PyodA(''Eco''RI)_F |
| - | | | + | |CCGGAATTCCTTCATATTGCCGACAAAGTACG |
|- | |- | ||
| - | | | + | |mAAA(''Spe''I)_R |
| - | | | + | |GGACTAGTTTATCACAGGGGCCGTCCG |
|- | |- | ||
| - | | | + | |PzntA(''Xba''I)_F |
| - | | | + | |GCTCTAGACGTCCGCTCGCTGTATCTC |
|- | |- | ||
| - | | | + | |RFP(''Pst''I)_R |
| - | | | + | |AACTGCAGCGGCCGCTACTAGTTTATTAAGCACCGGTGGAGTGA |
|- | |- | ||
| - | | | + | |ParsR(''Xba''I)_F |
| - | | | + | |GCTCTAGACCAACTCAAAATTCACACCTATTAC |
|- | |- | ||
| - | | | + | |GFP(''Pst''I)_R |
| - | | | + | |AACTGCAGTTAAGGCCTTTTGTATAGTTCATCC |
|} | |} | ||
| - | + | ||
== [ Preparation of competent cells ] 2010-09-01 ~ 2010-09-03 == | == [ Preparation of competent cells ] 2010-09-01 ~ 2010-09-03 == | ||
<br/> | <br/> | ||
| Line 82: | Line 78: | ||
2. Preparation of 200mL 2x LB broth, TSS solution and LB plates with ampicillin(100μg/mL) and chloramphenicol(25μg /mL), respectively | 2. Preparation of 200mL 2x LB broth, TSS solution and LB plates with ampicillin(100μg/mL) and chloramphenicol(25μg /mL), respectively | ||
| - | 3. Inoculation of subcultured E. coli to 200mL 2x LB borth | + | 3. Inoculation of subcultured ''E. coli'' to 200mL 2x LB borth |
4. Preparation of competent cells by CSBL laboratory protocol | 4. Preparation of competent cells by CSBL laboratory protocol | ||
| Line 112: | Line 108: | ||
2. 20uL suspension by autoclaved distilled water | 2. 20uL suspension by autoclaved distilled water | ||
| - | 3. 3uL transformation to E. coli DH5α | + | 3. 3uL transformation to ''E. coli'' DH5α |
4. Plating to LB(Amp100), LB(Cm25) | 4. Plating to LB(Amp100), LB(Cm25) | ||
| Line 121: | Line 117: | ||
1. Inoculation for plasmid DNA purification | 1. Inoculation for plasmid DNA purification | ||
| - | 2. E. coli K12 genomic DNA extraction by AccuPrep® Genomic DNA Extraction Kit | + | 2. ''E. coli'' K12 genomic DNA extraction by AccuPrep® Genomic DNA Extraction Kit |
3. Confirmation of genomic DNA by agarose gel electrophoresis (Figure 1) | 3. Confirmation of genomic DNA by agarose gel electrophoresis (Figure 1) | ||
| Line 127: | Line 123: | ||
4. Quantification of DNA concentration by NanoDrop : 137.5ng/μL | 4. Quantification of DNA concentration by NanoDrop : 137.5ng/μL | ||
| - | <br | + | |
| - | <br | + | [[Image:Real_figure_1.jpg|left|140px|frame|figure1. lane1;M-lane2;''E. coli'' K12 genomic DNA extraction]] |
| + | |||
| + | |||
| + | <br><br><br><br><br><br><br><br><br>br> | ||
| + | <br><br><br><br><br><br><br><br><br><br> | ||
| + | <br><br><br><br><br><br><br><br><br><br> | ||
| + | <br><br><br><br><br><br><br><br><br><br> | ||
| + | |||
== [ Plasmid DNA extraction : pSB1A3 and pSB1C3 ] 2010-09-07== | == [ Plasmid DNA extraction : pSB1A3 and pSB1C3 ] 2010-09-07== | ||
<br/> | <br/> | ||
| Line 136: | Line 139: | ||
3. Quantification of DNA concentration by NanoDrop | 3. Quantification of DNA concentration by NanoDrop | ||
| + | [[Image:Figure1_실험.png |250px|left|frame|figure2.'''lane1;M-lane2;pSB1A2-lane3;pSB1C3''']] | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | <br><br> | ||
| + | <br><br><br> | ||
| + | <br> | ||
| + | <br><br> | ||
| + | <br> | ||
| + | <br><br> | ||
| + | <br> | ||
| + | <br> | ||
| + | <br> | ||
| + | <br> | ||
| + | <br> | ||
| + | <br> | ||
| + | <br> | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
<br/> | <br/> | ||
<br/> | <br/> | ||
| + | |||
== [ PCR : promoters and reporter genes ] 2010-09-13 ~ 2010-09-16== | == [ PCR : promoters and reporter genes ] 2010-09-13 ~ 2010-09-16== | ||
<br/> | <br/> | ||
| - | 1. PCR : | + | 1. PCR : PyodA-mAAA, PzntA-RFP(BBa_E1010) and ParsR-GFP(BBa_E0040) |
<br/> | <br/> | ||
{| border="2" | {| border="2" | ||
| Line 172: | Line 217: | ||
4. Quantification of DNA concentration by NanoDrop | 4. Quantification of DNA concentration by NanoDrop | ||
| + | |||
| + | |||
| + | [[Image:Figure2 실험.png|140px|left|frame|figure3.lane1;M-lane2;(PyodA-mAAA)-lane3;(Pznt-RFP)-lane4;(ParsR-GFP)]] | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | <br/><br/><br/><br/><br/><br/> | ||
| + | <br/><br/><br/><br/><br/><br/> | ||
| + | <br/><br/><br/><br/><br/><br/> | ||
| + | <br/><br/><br/><br/><br/><br/> | ||
<br/> | <br/> | ||
<br/> | <br/> | ||
| + | <br/> | ||
| + | <br/><br/> | ||
| + | <br/><br/><br/><br/><br/><br/><br/> | ||
| + | |||
== [ Digestion] 2010-09-17 == | == [ Digestion] 2010-09-17 == | ||
1. Digestion of PCR products and pSB1A2 | 1. Digestion of PCR products and pSB1A2 | ||
| - | :1) | + | :1) PyodA-mAAA : ''Eco''RI and ''Spe''I |
| - | :2) | + | :2) PzntA-RFP : ''Xba''I and ''Pst''I |
| - | :3) pSB1A3 : | + | :3) pSB1A3 : ''Eco''RI and ''Pst''I |
<br/> | <br/> | ||
{| border="2" | {| border="2" | ||
| Line 209: | Line 272: | ||
3. Quantification of DNA concentration by NanoDrop | 3. Quantification of DNA concentration by NanoDrop | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | [[Image:4 Figure3 실험.png|left|140px|frame|figure4.M pSB1A2(''Eco''RI/''Pst''I) PyodA-mAAA(''Eco''RI/''Spe''I) PzntA-RFP(''Xba''I/''Pst''I)]] | ||
| + | |||
| + | |||
<br/> | <br/> | ||
<br/> | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | |||
| + | <br/> | ||
| + | <br/> | ||
| + | |||
| + | <br/> | ||
| + | <br/> | ||
| + | |||
| + | <br/> | ||
| + | <br/> | ||
| + | |||
| + | <br/> | ||
| + | <br/> | ||
| + | |||
| + | <br/> | ||
| + | <br/> | ||
| + | |||
| + | <br/> | ||
| + | <br/> | ||
| + | |||
| + | <br/> | ||
| + | <br/> | ||
| + | |||
| + | <br/> | ||
| + | <br/> | ||
| + | |||
== [Chuseok, Korean thanksgiving day] 2010-09-20 ~ 2010-09== | == [Chuseok, Korean thanksgiving day] 2010-09-20 ~ 2010-09== | ||
<br/> | <br/> | ||
| Line 257: | Line 373: | ||
3. Recombinant plasmid sequencing by COSMO GeneTech | 3. Recombinant plasmid sequencing by COSMO GeneTech | ||
| - | <br | + | [[Image:5_Figure4_실험.png|left|140px|frame|figure5. lane1;M-lane2; (pSB1A2)-[PyodA-mAA-Pznt-RFP] #1,2,3]] |
| - | <br | + | |
| + | <br><br> | ||
| + | <br><br><br><br><br><br><br><br><br><br> | ||
| + | <br><br> | ||
| + | <br><br> | ||
| + | <br><br> | ||
| + | <br><br> | ||
| + | <br><br><br><br> | ||
| + | <br><br> | ||
| + | <br><br> | ||
| + | <br><br> | ||
| + | <br><br><br><br><br><br> | ||
| + | |||
== [ Digestion ] 2010-10-01 ~ 2010-10-03== | == [ Digestion ] 2010-10-01 ~ 2010-10-03== | ||
<br/> | <br/> | ||
1. Check : recombinant plasmid sequence | 1. Check : recombinant plasmid sequence | ||
2. Selection of correct clones | 2. Selection of correct clones | ||
| - | 3. Digestion of PCR products( | + | 3. Digestion of PCR products(ParsR-GFP) and pSB1C3 |
| - | :1) '' | + | :1) PyodA-mAAA-PzntA-RFP : ''Eco''RI and ''Spe''I |
| - | : | + | :2) ParsR-GFP : ''Eco''RI and ''Spe''I |
| + | :3) pSB1C3 : ''Eco''RI and ''Pst''I | ||
<br/> | <br/> | ||
{| border="2" | {| border="2" | ||
| Line 291: | Line 420: | ||
<br/> | <br/> | ||
4. Confirmation of digested products by agarose gel electrophoresis (Figure 6) | 4. Confirmation of digested products by agarose gel electrophoresis (Figure 6) | ||
| + | [[Image:Figure6 aaa.png|left|140px|frame|figure6. lane1; M-lane2; (PyodA-mAAA-PzntA-RFP(EcoRI/SpeI))-lane3; (ParsR-GFP(XbaI/SpeI))-lane4; (pSB1C3(EcoRI/PstI))]] | ||
| + | |||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | |||
| + | |||
5. Quantification of DNA concentration by NanoDrop | 5. Quantification of DNA concentration by NanoDrop | ||
| - | 6. Ligation of each parts : | + | 6. Ligation of each parts : PyodA-mAAA-PzntA-RFP, ParsR-GFP and pSB1C3 |
<br/> | <br/> | ||
| Line 320: | Line 478: | ||
<br/> | <br/> | ||
<br/> | <br/> | ||
| + | |||
== [ Confirmation of 2nd cloning ] 2010-10-06== | == [ Confirmation of 2nd cloning ] 2010-10-06== | ||
<br/> | <br/> | ||
| Line 327: | Line 486: | ||
<br/> | <br/> | ||
<br/> | <br/> | ||
| - | == [ Plasmid DNA extraction : pSB1C3-( | + | == [ Plasmid DNA extraction : pSB1C3-( PyodA-mAAA-PzntA-RFP-ParsR-GFP) ] 2010-10-07== |
<br/> | <br/> | ||
1. Plasmid DNA purification by LaboPass™ Plasmid Mini | 1. Plasmid DNA purification by LaboPass™ Plasmid Mini | ||
| Line 334: | Line 493: | ||
3. Recombinant plasmid full-sequencing by COSMO GeneTech | 3. Recombinant plasmid full-sequencing by COSMO GeneTech | ||
| + | |||
| + | [[Image:Figure7_실험.png|left|100px|frame|figure7. lane1;M-lane2; (psB1A2) Heavy-metal detector #1~3]] | ||
| + | |||
<br/> | <br/> | ||
<br/> | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <br/> | ||
| + | |||
== [ Completion : Heavy-metal detector ] 2010-10-18== | == [ Completion : Heavy-metal detector ] 2010-10-18== | ||
<br/> | <br/> | ||
Latest revision as of 03:58, 28 October 2010

Brain storming & Work notes
Click on a date to see notes on the meeting & summary of labwork done on that day.
|
|
|
|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Experimental notes
[Discussion] 2010-08-02 ~ 2010-08-29
1. Strategy and overview of iGEM 2010 experiment
2. Design of primers
| Primer | Sequence ( 5’ → 3’ ) |
|---|---|
| PyodA(EcoRI)_F | CCGGAATTCCTTCATATTGCCGACAAAGTACG |
| mAAA(SpeI)_R | GGACTAGTTTATCACAGGGGCCGTCCG |
| PzntA(XbaI)_F | GCTCTAGACGTCCGCTCGCTGTATCTC |
| RFP(PstI)_R | AACTGCAGCGGCCGCTACTAGTTTATTAAGCACCGGTGGAGTGA |
| ParsR(XbaI)_F | GCTCTAGACCAACTCAAAATTCACACCTATTAC |
| GFP(PstI)_R | AACTGCAGTTAAGGCCTTTTGTATAGTTCATCC |
[ Preparation of competent cells ] 2010-09-01 ~ 2010-09-03
1. Inoculation of E. coli DH5α and E. coli BL21(DE3) to 3mL LB broth
2. Preparation of 200mL 2x LB broth, TSS solution and LB plates with ampicillin(100μg/mL) and chloramphenicol(25μg /mL), respectively
3. Inoculation of subcultured E. coli to 200mL 2x LB borth
4. Preparation of competent cells by CSBL laboratory protocol
5. Transformation of pUC19 plasmid(10ng/μL) to competent cells for transformation efficiency check
[ Transformation efficiency ] 2010-09-04
| Strain | Number of colonies (colonies/μg DNA) |
|---|---|
| E. coli DH5α | Number of colonies (colonies/μg DNA) |
| E. coli BL21(DE3) | 1.5 x 105 |
[ Amplification of BioBrick parts : pSB1A2 and pSB1C3 ] 2010-09-05
1. Confirmed location : pSB1A2-BBa_E0040 (2010 Kit plate 1/ 14K) and pSB1C3-BBa_J04450 (2010 Kit plate 1/ 3A)
2. 20uL suspension by autoclaved distilled water
3. 3uL transformation to E. coli DH5α
4. Plating to LB(Amp100), LB(Cm25)
[ Genomic DNA extraction ] 2010-09-06
1. Inoculation for plasmid DNA purification
2. E. coli K12 genomic DNA extraction by AccuPrep® Genomic DNA Extraction Kit
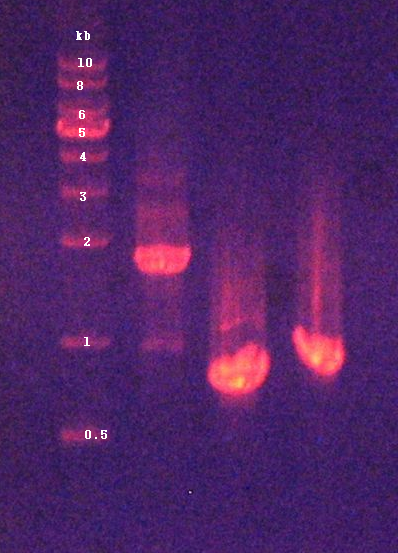
3. Confirmation of genomic DNA by agarose gel electrophoresis (Figure 1)
4. Quantification of DNA concentration by NanoDrop : 137.5ng/μL
br>
[ Plasmid DNA extraction : pSB1A3 and pSB1C3 ] 2010-09-07
1. Plasmid miniprep by LaboPass™ Plasmid Mini (Plasmid DNA purification kit)
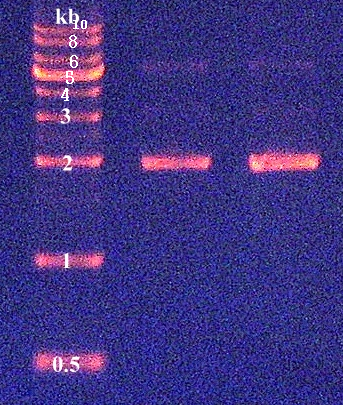
2. Confirmation of extracted plasmids by agarose gel electrophoresis (Figure 2)
3. Quantification of DNA concentration by NanoDrop
[ PCR : promoters and reporter genes ] 2010-09-13 ~ 2010-09-16
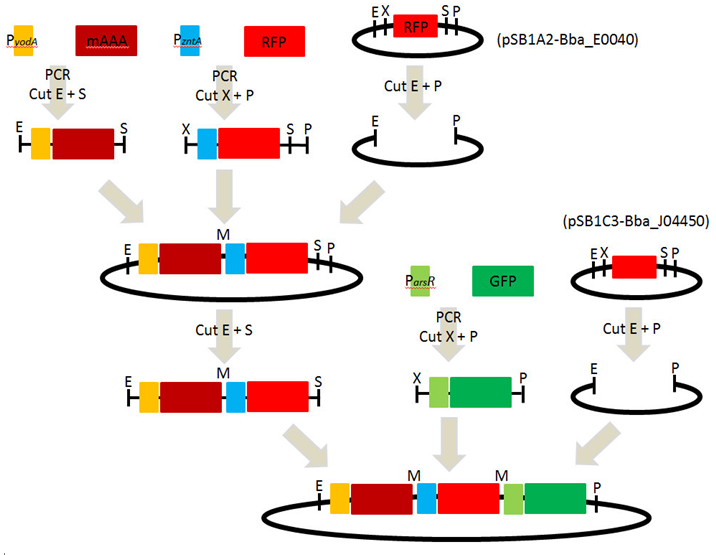
1. PCR : PyodA-mAAA, PzntA-RFP(BBa_E1010) and ParsR-GFP(BBa_E0040)
| Reagent | Volume (μL) |
|---|---|
| 2.5mM dNTP | 3 |
| 10x buffer | 5 |
| Plasmid template (20ng/μL) | 2 |
| Primers (10pmole/μL) | 4 |
| α-Taq DNA polymerase (5U/μL) | 0.5 |
| D.W. | 35.5/total=50 |
| 95˚C(2’)-[95˚C(20”)-55˚C(20”)-72˚C(2’)]30-72˚C(5’)-4˚C |
2. Confirmation of PCR products by agarose gel electrophoresis (Figure 3)
3. Purified PCR products
4. Quantification of DNA concentration by NanoDrop
[ Digestion] 2010-09-17
1. Digestion of PCR products and pSB1A2
- 1) PyodA-mAAA : EcoRI and SpeI
- 2) PzntA-RFP : XbaI and PstI
- 3) pSB1A3 : EcoRI and PstI
| Reagent | Volume (μL) |
|---|---|
| DNA (about 30ng/μL) | 30 |
| 10x NEB buffer 2 | 5 |
| BSA (10mg/mL) | 0.5 |
| Appropriate 1st and 2nd restriction enzymes | 2 (each 1) |
| D.W. | 12.5 / total = 50 |
| Completely digestion at 37˚C for 2 hours (at least)
and stop at 80˚C for 20min |
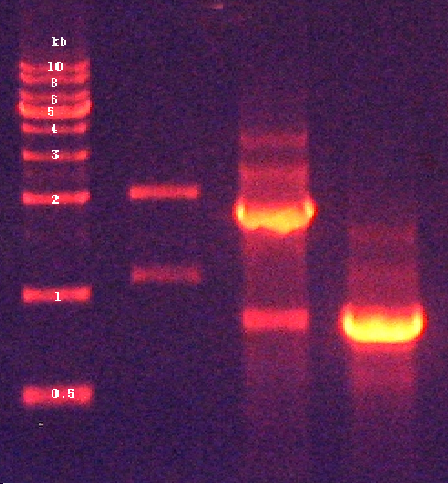
2. Confirmation of digested products by agarose gel electrophoresis (Figure 4)
3. Quantification of DNA concentration by NanoDrop
[Chuseok, Korean thanksgiving day] 2010-09-20 ~ 2010-09
[ Ligation & Transformation ] 2010-09-27
1. Ligation of each parts : PyodA-mAAA, PzntA-RFP and pSB1A2
| Reagent | Volume (μL) |
|---|---|
| 10x T4 DNA ligase reaction buffer | 2 |
| T4 DNA ligase | 2 |
| Each of the digests | 2 + 2 + 2 = 8 |
| D.W. | 8 / total = 20 |
| Incubation at room temperature for 30min
and stop at 80˚C for 20min |
2. Transformation to E. coli DH5α
[ Confirmation of 1st cloning ] 2010-09-28
1. Check : the color of colonies (pSB1A2 : green, recombinant plasmid : white)
2. Inoculation of white colonies to 3mL LB(Amp100)
[ Plasmid DNA extraction : pSB1A2-( PyodA-mAAA-PzntA-RFP) ] 2010-09-29
1. Plasmid DNA purification by LaboPass™ Plasmid Mini
2. Confirmation of extracted plasmids by agarose gel electrophoresis (Figure 5)
3. Recombinant plasmid sequencing by COSMO GeneTech
[ Digestion ] 2010-10-01 ~ 2010-10-03
1. Check : recombinant plasmid sequence
2. Selection of correct clones
3. Digestion of PCR products(ParsR-GFP) and pSB1C3
- 1) PyodA-mAAA-PzntA-RFP : EcoRI and SpeI
- 2) ParsR-GFP : EcoRI and SpeI
- 3) pSB1C3 : EcoRI and PstI
| Reagent | Volume (μL) |
|---|---|
| DNA (about 30ng/μL) | 30 |
| 10x NEB buffer 2 | 5 |
| BSA (10mg/mL) | 0.5 |
| Appropriate 1st and 2nd restriction enzymes | 2 (each 1) |
| D.W. | 12.5 / total = 50 |
| Completely digestion at 37˚C for 2 hours (at least)
and stop at 80˚C for 20min |
4. Confirmation of digested products by agarose gel electrophoresis (Figure 6)
5. Quantification of DNA concentration by NanoDrop
6. Ligation of each parts : PyodA-mAAA-PzntA-RFP, ParsR-GFP and pSB1C3
| Reagent | Volume (μL) |
|---|---|
| 10x T4 DNA ligase reaction buffer | 2 |
| T4 DNA ligase | 2 |
| Each of the digests | 2 + 2 + 2 = 8 |
| D.W. | 8 / total = 20 |
| Incubation at room temperature for 30min
and stop at 80˚C for 20min |
7. Transformation to E. coli DH5α
[ Confirmation of 2nd cloning ] 2010-10-06
1. Check : the color of colonies (pSB1C3 : red, recombinant plasmid : white)
2. Inoculation of white colonies to 3mL LB(Amp100)
[ Plasmid DNA extraction : pSB1C3-( PyodA-mAAA-PzntA-RFP-ParsR-GFP) ] 2010-10-07
1. Plasmid DNA purification by LaboPass™ Plasmid Mini
2. Confirmation of extracted plasmids by agarose gel electrophoresis (Figure 7)
3. Recombinant plasmid full-sequencing by COSMO GeneTech
[ Completion : Heavy-metal detector ] 2010-10-18
1. Check : recombinant plasmid sequence
2. Selection of correct clones
3. Transformation to E. coli BL21(DE3) for expression test
 "
"