Team:ETHZ Basel/Achievements/E lemming
From 2010.igem.org
(→Characterization) |
(→Simulation Results) |
||
| (20 intermediate revisions not shown) | |||
| Line 4: | Line 4: | ||
= The E. lemming = | = The E. lemming = | ||
| - | == What it needs to bring E. lemming alive == | + | == What it needs to bring the E. lemming alive == |
| - | It needs an '''archeal photoreceptor''' that is fused to a '''bacterial chemotactic transducer'''. | + | It needs an '''archeal photoreceptor''' that is fused to a '''bacterial chemotactic transducer''' ([http://partsregistry.org/Part:BBa_K422001 BBa_K422001]). |
| - | This was successfully demonstrated by Jung ''et al.'' in 2001, who fused the ''Natronobacterium pharaonis'' NpSRII (Np seven-transmembrane retinylidene photoreceptor sensory rhodopsins II) and their cognate transducer HtrII to the cytoplasmic domain of the chemotaxis transducer EcTsr of ''Escherichia coli''. For more information visit our [[Team:ETHZ_Basel/Biology/Archeal_Light_Receptor|Archeal Light Receptor]] | + | This was successfully demonstrated by Jung ''et al.'' in 2001, who fused the ''Natronobacterium pharaonis'' NpSRII (Np seven-transmembrane retinylidene photoreceptor sensory rhodopsins II) and their cognate transducer HtrII to the cytoplasmic domain of the chemotaxis transducer EcTsr of ''Escherichia coli''. For more information visit our [[Team:ETHZ_Basel/Biology/Archeal_Light_Receptor|Archeal Light Receptor]] page. |
To make the nice videos shown below, the optimal chemotactic conditions, that were concluded from a [[Team:ETHZ_Basel/Biology/Implementation|series of different microscopy images]], were applied. ''Escherichia coli'' K12 cells were grown at 30 °C in Lysogeny Broth to on OD of 1.0. IPTG for induction of gene expression and all-trans retinal for NpSRII folding were added to the media. | To make the nice videos shown below, the optimal chemotactic conditions, that were concluded from a [[Team:ETHZ_Basel/Biology/Implementation|series of different microscopy images]], were applied. ''Escherichia coli'' K12 cells were grown at 30 °C in Lysogeny Broth to on OD of 1.0. IPTG for induction of gene expression and all-trans retinal for NpSRII folding were added to the media. | ||
== Experimental Results == | == Experimental Results == | ||
| - | We imaged several transfected E. coli cells with a 20× lens in a ≈50μm high flow channel. Approximately 5% of the cells reacted on the switch-on and -off of the blue light signal by changing significantly their swimming behavior. | + | We imaged several transfected ''E. coli'' cells with a 20× lens in a ≈50μm high flow channel. Approximately 5% of the cells reacted on the switch-on and -off of the blue light (≈500 nm) signal by changing significantly their swimming behavior. Video 1 shows an E. lemming swimming in regular circles in a constant light environment. When switching the blue light on, it completely changes its motility after a 2-3s delay by swimming straight for several seconds. When the light is switched off, it returns to its original behavior after a similar delay (see paragraph [[Team:ETHZ_Basel/Achievements/E_lemming#Characterization|"Characterization"]]). |
Video 2 shows another E. lemming which is swimming straight with frequent interruptions by tumblings when being in a constant light environment. When the blue light is switched on, the tumblings nearly completely disappear and the E. lemming is swimming straight over large distances. When the light is switched off, the tumbling disappears or the E. lemming alternatively stops movement at all. | Video 2 shows another E. lemming which is swimming straight with frequent interruptions by tumblings when being in a constant light environment. When the blue light is switched on, the tumblings nearly completely disappear and the E. lemming is swimming straight over large distances. When the light is switched off, the tumbling disappears or the E. lemming alternatively stops movement at all. | ||
| - | + | ||
| + | <html><table width="90%" border="0"><tr> | ||
<td valign="top" style="width:50%"> | <td valign="top" style="width:50%"> | ||
<div class="thumb tright"><div class="thumbinner" style="width:402px;"> | <div class="thumb tright"><div class="thumbinner" style="width:402px;"> | ||
<iframe title="YouTube video player" class="youtube-player" type="text/html" width="400" height="325" src="http://www.youtube.com/embed/mulRvAVExSc?rel=0&hd=1" frameborder="0"></iframe> | <iframe title="YouTube video player" class="youtube-player" type="text/html" width="400" height="325" src="http://www.youtube.com/embed/mulRvAVExSc?rel=0&hd=1" frameborder="0"></iframe> | ||
| - | <div class="thumbcaption"><div class="magnify"><a href="http://www.youtube.com/watch?v=mulRvAVExSc&hd=1" class="external" title="Enlarge"><img src="/wiki/skins/common/images/magnify-clip.png" width="15" height="11" alt="" /></a></div><b>Video 1: | + | <div class="thumbcaption"><div class="magnify"><a href="http://www.youtube.com/watch?v=mulRvAVExSc&hd=1" class="external" title="Enlarge"><img src="/wiki/skins/common/images/magnify-clip.png" width="15" height="11" alt="" /></a></div><b>Video 1: The E. lemming in action!</b><br/> |
| + | <i>Blue dots</i>: the detected <i>E. coli</i> cells. <i>Yellow dot</i>: the currently selected E. lemming. <i>Yellow cone</i>: the current swimming direction of E. lemming. <i>Yellow dotted line</i>: the path of the E. lemming. <i>Blue background</i>: blue light on (inducing directed movement). <i>Gray environment</i>: blue light off (inducing tumbling).</br> | ||
| + | <br> Note how the E. lemming is keeping its direction under the influence of blue light, whereas it is tumbling and quickly changing directions when the blue light is off. </br> | ||
| + | <br>The unprocessed microscope images are available <a href="https://2010.igem.org/Team:ETHZ_Basel/Achievements/OriginalImages">here</a>.</div></div></div> | ||
</td> | </td> | ||
<td valign="top" style="width:50%"> | <td valign="top" style="width:50%"> | ||
<html><div class="thumb tright"><div class="thumbinner" style="width:402px;"> | <html><div class="thumb tright"><div class="thumbinner" style="width:402px;"> | ||
| - | <iframe title="YouTube video player" class="youtube-player" type="text/html" width="400" height="325" src="http://www.youtube.com/embed/ | + | <iframe title="YouTube video player" class="youtube-player" type="text/html" width="400" height="325" src="http://www.youtube.com/embed/teiz5r0mzi0?rel=0&hd=1" frameborder="0"></iframe> |
| - | <div class="thumbcaption"><div class="magnify"><a href="http://www.youtube.com/watch?v= | + | <div class="thumbcaption"><div class="magnify"><a href="http://www.youtube.com/watch?v=teiz5r0mzi0&hd=1" class="external" title="Enlarge"><img src="/wiki/skins/common/images/magnify-clip.png" width="15" height="11" alt="" /></a></div><b>Video 2: E. lemming's brother in action!</b> The brother of the E. lemming decided to swim several times nearly out of focus and out of the field of view such that he had to be tracked manually</b>.<br /> |
| + | <i>Blue dots</i>: the detected <i>E. coli</i> cells. <i>Yellow dot</i>: the currently selected E. lemming. <i>Yellow cone</i>: the current swimming direction of E. lemming. <i>Yellow dotted line</i>: the path of the E. lemming. <i>Red arrow</i>: the direction of the microscope shift in order to place the E. lemming in the center of the frame <i>Blue background</i>: blue light on (inducing directed movement). <i>Gray environment</i>: blue light off (inducing tumbling).</br> | ||
| + | <br> Note how the E. lemming is keeping its direction under the influence of blue light, whereas it is tumbling and quickly changing directions when the blue light is off. </br> | ||
| + | <br>The unprocessed microscope images are available <a href="https://2010.igem.org/Team:ETHZ_Basel/Achievements/OriginalImages">here</a>.</div></div></div> | ||
</td></tr></table> | </td></tr></table> | ||
</html> | </html> | ||
| Line 31: | Line 38: | ||
To characterize the change of swimming behavior when switching on or off the blue light signal, we estimated the angle of the E. lemming for each frame of Video 1. This was done by obtaining the positions (x<sub>i</sub>, y<sub>i</sub>) of the E. lemming from our [[Team:ETHZ_Basel/InformationProcessing/CellDetection|cell detection and tracking algorithm]]. The angle φ<sub>i</sub> of frame i was then calculated by central differences:<br /> | To characterize the change of swimming behavior when switching on or off the blue light signal, we estimated the angle of the E. lemming for each frame of Video 1. This was done by obtaining the positions (x<sub>i</sub>, y<sub>i</sub>) of the E. lemming from our [[Team:ETHZ_Basel/InformationProcessing/CellDetection|cell detection and tracking algorithm]]. The angle φ<sub>i</sub> of frame i was then calculated by central differences:<br /> | ||
<code>tan(φ<sub>i</sub>)=(y<sub>i+1</sub>-y<sub>i-1</sub>)/(x<sub>i+1</sub>-x<sub>i-1</sub>).</code><br /> | <code>tan(φ<sub>i</sub>)=(y<sub>i+1</sub>-y<sub>i-1</sub>)/(x<sub>i+1</sub>-x<sub>i-1</sub>).</code><br /> | ||
| - | [[Image:AngleOverTime.jpg|thumb|center|900px|'''Figure 1: Angle of the E. lemming''' during one measurement (see Video 1) as calculated from the central differences of its positions. The estimated reaction times between the switching | + | [[Image:AngleOverTime.jpg|thumb|center|900px|'''Figure 1: Angle of the E. lemming''' during one measurement (see Video 1) as calculated from the central differences of its positions. The estimated reaction times between the switching off the blue light and the reactions of the E. lemming are marked in the image. For the reaction delay between switch-on of the light and straight swimming we obtained Δt<sub>1</sub>≈2.1s and Δt<sub>2</sub>≈3.0s. For the delay between the switch-off of the blue light and start of tumbling it was only possible to estimate the time delay for the second light pulse, Δt<sub>3</sub>≈2.4s. White background: blue light off. Light blue background: blue light on.]] |
| - | When plotting the angle over time (see Figure 1), one observes that during white light periods the angle is increasing with a nearly constant angular speed of about 27° per second (& | + | When plotting the angle over time (see Figure 1), one observes that during white light periods the angle is increasing with a nearly constant angular speed of about 27° per second (≈8° per frame). When switching on blue light, the angular speed decreases to nearly zero for several seconds after a delay between 2 and 3s. |
For the first light pulse this decrease of angular speed lasted for about 10s until the return to pre-blue light behavior, for the second light pulse this effect only ended after the blue light was switched off again. In the latter, normal swimming behavior re-established after a delay of approximately 2.4s, which is nearly the same delay as the delay when switching the light on. | For the first light pulse this decrease of angular speed lasted for about 10s until the return to pre-blue light behavior, for the second light pulse this effect only ended after the blue light was switched off again. In the latter, normal swimming behavior re-established after a delay of approximately 2.4s, which is nearly the same delay as the delay when switching the light on. | ||
| Line 38: | Line 45: | ||
We furthermore noticed that the E. lemming seems to have the tendency to show a bigger tumble right before starting swimming straight when the blue light is switched on. However, if this behavior occurred by chance or if this is a general property of the E. lemming was yet not possible to show. | We furthermore noticed that the E. lemming seems to have the tendency to show a bigger tumble right before starting swimming straight when the blue light is switched on. However, if this behavior occurred by chance or if this is a general property of the E. lemming was yet not possible to show. | ||
| + | |||
| + | == Simulation Results == | ||
| + | To get a deeper understanding of the underlying network dynamics, we built a [[Team:ETHZ_Basel/Modeling/Light_Switch#Archeal light receptor|molecular model of the archeal photoreceptor]]. It shows similar behavior to blue light pulses as experimentally observed (see Figure 2): After blue light is shut-on, the bias (probability the cell is swimming straight) is increasing rapidly with a time constant of about 1.5s. When the blue light stays on, the bias is slowly decreasing over time, such that the probability for falling back in tumbling behavior increases as longer the light stays switched on, due to the adaptation of the chemotaxis pathway. When switching the light off, the bias is decrasing with a similar time constant as when switching on the blue light. | ||
| + | [[Image:simBiasOverTime.jpg|thumb|center|900px|'''Figure 2: Bias of the E. lemming''' as predicted by our mathematical model. The bias is the probability of a cell to swim straight. This plot was generated by applying the same light pulses as used experimentally (see Figure 1). Red: bias. Blue: CheYp concentration. Green: photoreceptor occupancy.]] | ||
| + | The predicted delay between the change of light intensity and a change in swimming behavior of the E. lemming is approximately 0.5-1s slower than experimentally measured. We believe that this difference might be due to the several dynamic processes not explicitly modeled by us, like the traveling time of the phosphorylated CheY proteins between the receptor and the motor complexes and the reaction time of the flagella. | ||
Latest revision as of 00:29, 28 October 2010
The E. lemming
What it needs to bring the E. lemming alive
It needs an archeal photoreceptor that is fused to a bacterial chemotactic transducer ([http://partsregistry.org/Part:BBa_K422001 BBa_K422001]). This was successfully demonstrated by Jung et al. in 2001, who fused the Natronobacterium pharaonis NpSRII (Np seven-transmembrane retinylidene photoreceptor sensory rhodopsins II) and their cognate transducer HtrII to the cytoplasmic domain of the chemotaxis transducer EcTsr of Escherichia coli. For more information visit our Archeal Light Receptor page.
To make the nice videos shown below, the optimal chemotactic conditions, that were concluded from a series of different microscopy images, were applied. Escherichia coli K12 cells were grown at 30 °C in Lysogeny Broth to on OD of 1.0. IPTG for induction of gene expression and all-trans retinal for NpSRII folding were added to the media.
Experimental Results
We imaged several transfected E. coli cells with a 20× lens in a ≈50μm high flow channel. Approximately 5% of the cells reacted on the switch-on and -off of the blue light (≈500 nm) signal by changing significantly their swimming behavior. Video 1 shows an E. lemming swimming in regular circles in a constant light environment. When switching the blue light on, it completely changes its motility after a 2-3s delay by swimming straight for several seconds. When the light is switched off, it returns to its original behavior after a similar delay (see paragraph "Characterization").
Video 2 shows another E. lemming which is swimming straight with frequent interruptions by tumblings when being in a constant light environment. When the blue light is switched on, the tumblings nearly completely disappear and the E. lemming is swimming straight over large distances. When the light is switched off, the tumbling disappears or the E. lemming alternatively stops movement at all.
|
Video 1: The E. lemming in action! Blue dots: the detected E. coli cells. Yellow dot: the currently selected E. lemming. Yellow cone: the current swimming direction of E. lemming. Yellow dotted line: the path of the E. lemming. Blue background: blue light on (inducing directed movement). Gray environment: blue light off (inducing tumbling). Note how the E. lemming is keeping its direction under the influence of blue light, whereas it is tumbling and quickly changing directions when the blue light is off. The unprocessed microscope images are available here. |
Video 2: E. lemming's brother in action! The brother of the E. lemming decided to swim several times nearly out of focus and out of the field of view such that he had to be tracked manually. Blue dots: the detected E. coli cells. Yellow dot: the currently selected E. lemming. Yellow cone: the current swimming direction of E. lemming. Yellow dotted line: the path of the E. lemming. Red arrow: the direction of the microscope shift in order to place the E. lemming in the center of the frame Blue background: blue light on (inducing directed movement). Gray environment: blue light off (inducing tumbling). Note how the E. lemming is keeping its direction under the influence of blue light, whereas it is tumbling and quickly changing directions when the blue light is off. The unprocessed microscope images are available here. |
Characterization
To characterize the change of swimming behavior when switching on or off the blue light signal, we estimated the angle of the E. lemming for each frame of Video 1. This was done by obtaining the positions (xi, yi) of the E. lemming from our cell detection and tracking algorithm. The angle φi of frame i was then calculated by central differences:
tan(φi)=(yi+1-yi-1)/(xi+1-xi-1).
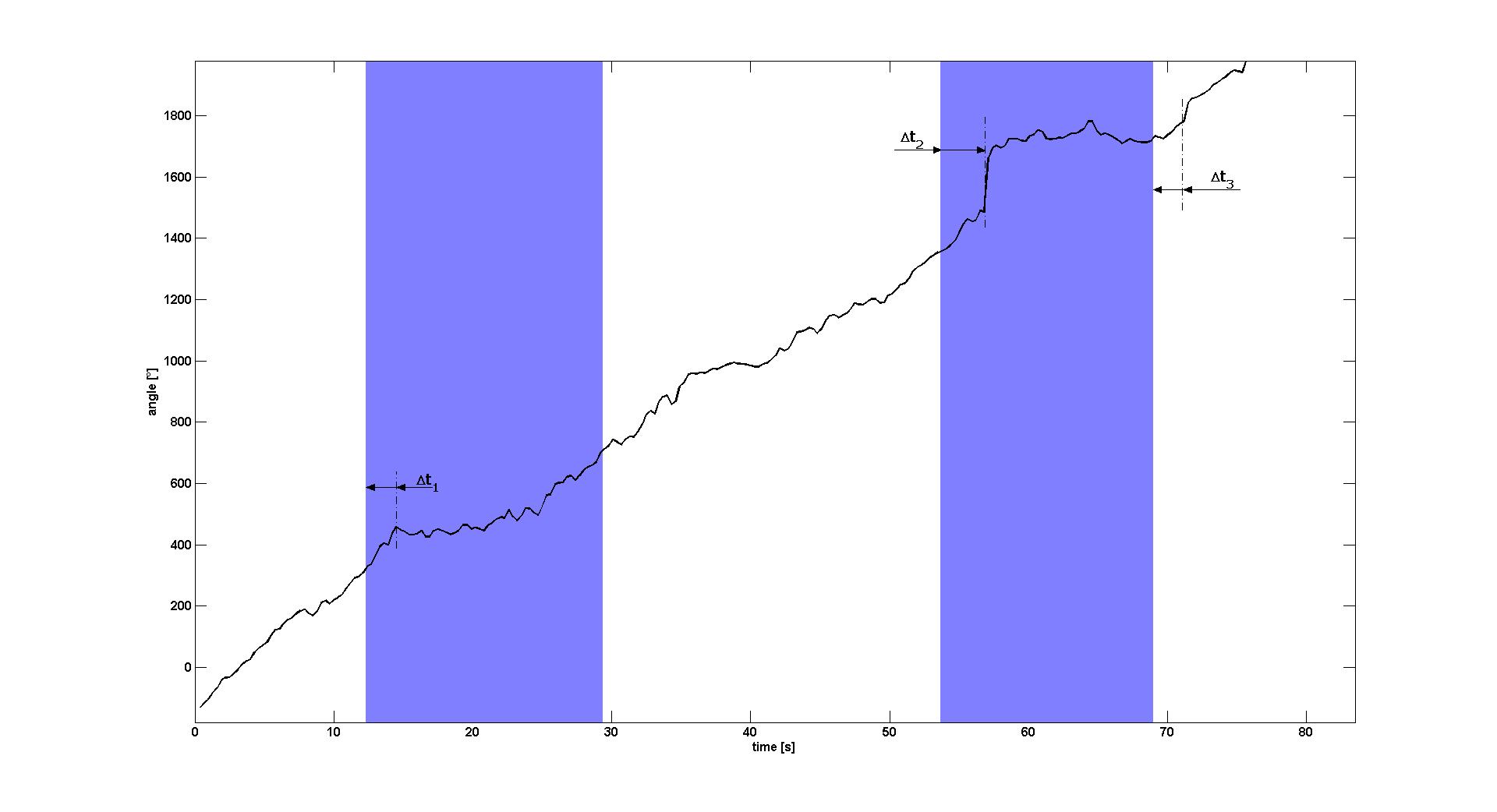
When plotting the angle over time (see Figure 1), one observes that during white light periods the angle is increasing with a nearly constant angular speed of about 27° per second (≈8° per frame). When switching on blue light, the angular speed decreases to nearly zero for several seconds after a delay between 2 and 3s.
For the first light pulse this decrease of angular speed lasted for about 10s until the return to pre-blue light behavior, for the second light pulse this effect only ended after the blue light was switched off again. In the latter, normal swimming behavior re-established after a delay of approximately 2.4s, which is nearly the same delay as the delay when switching the light on. Please note that the natural adaptation system of the chemotaxis pathway downstream of the light receptor is active in this mutant, such that the swimming behavior only changes directly after the blue light is switched on or off, but is not necessarily different between long periods of light on or off.
We furthermore noticed that the E. lemming seems to have the tendency to show a bigger tumble right before starting swimming straight when the blue light is switched on. However, if this behavior occurred by chance or if this is a general property of the E. lemming was yet not possible to show.
Simulation Results
To get a deeper understanding of the underlying network dynamics, we built a molecular model of the archeal photoreceptor. It shows similar behavior to blue light pulses as experimentally observed (see Figure 2): After blue light is shut-on, the bias (probability the cell is swimming straight) is increasing rapidly with a time constant of about 1.5s. When the blue light stays on, the bias is slowly decreasing over time, such that the probability for falling back in tumbling behavior increases as longer the light stays switched on, due to the adaptation of the chemotaxis pathway. When switching the light off, the bias is decrasing with a similar time constant as when switching on the blue light.
The predicted delay between the change of light intensity and a change in swimming behavior of the E. lemming is approximately 0.5-1s slower than experimentally measured. We believe that this difference might be due to the several dynamic processes not explicitly modeled by us, like the traveling time of the phosphorylated CheY proteins between the receptor and the motor complexes and the reaction time of the flagella.
 "
"



