SsrA Experiment
From 2010.igem.org
(→2010-07-31) |
(→YFP) |
||
| (18 intermediate revisions not shown) | |||
| Line 26: | Line 26: | ||
|E0240||876bp||1114bp | |E0240||876bp||1114bp | ||
|- | |- | ||
| - | |E0240+ | + | |E0240+LVA||909bp||1147bp |
|- | |- | ||
| - | |R0010+E0240+ | + | |R0010+E0240+LVA||1117bp||1355bp |
|} | |} | ||
| Line 51: | Line 51: | ||
|I13507||861bp||1099bp | |I13507||861bp||1099bp | ||
|- | |- | ||
| - | |I13507+ | + | |I13507+LVA||894bp||1132bp |
|- | |- | ||
| - | |R0010+I13507+ | + | |R0010+I13507+LVA||1102bp||1340bp |
|} | |} | ||
| Line 59: | Line 59: | ||
'''Purpose''' | '''Purpose''' | ||
| - | After ligate CFPlva to Terminator and RBS , ligate to pLac. | + | 1.After ligate CFPlva to Terminator and RBS , ligate to pLac. |
| + | |||
| + | 2.Remove LVA from the sequences to construct the control. | ||
'''Material''' | '''Material''' | ||
| Line 89: | Line 91: | ||
'''Purpose''' | '''Purpose''' | ||
| - | After ligate YFPlva to Terminator and RBS , ligate to pLac. | + | 1.After ligate YFPlva to Terminator and RBS , ligate to pLac. |
| + | |||
| + | 2.Remove LVA from the sequences to construct the control. | ||
'''Material''' | '''Material''' | ||
| Line 144: | Line 148: | ||
4.Run the gel for B0015. | 4.Run the gel for B0015. | ||
| - | + | ||
| - | + | [[image:NYMU_G0723.jpg]] | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
5.Measured the nano drop. | 5.Measured the nano drop. | ||
| Line 169: | Line 162: | ||
9.PCR to make GFPlva and RFPlva front parts. | 9.PCR to make GFPlva and RFPlva front parts. | ||
| - | + | ||
| - | + | [[image:0723.jpg]] | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
==2010-07-24== | ==2010-07-24== | ||
| Line 187: | Line 169: | ||
2.Run the gel. | 2.Run the gel. | ||
| - | + | ||
| - | + | [[Image:0724.jpg]] | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
3.PCR to make the whole parts GFPlva and RFPlva. | 3.PCR to make the whole parts GFPlva and RFPlva. | ||
4.Run the gel. | 4.Run the gel. | ||
| - | + | ||
| - | + | [[Image:0724 2.jpg]] | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
5.Digest GFPlva(XP) and RFPlva(XP). | 5.Digest GFPlva(XP) and RFPlva(XP). | ||
| Line 391: | Line 352: | ||
[[image:3165.jpg]] | [[image:3165.jpg]] | ||
| - | |||
5.Purify GFPlva(XP), RBS+YFPlva+term(XP) | 5.Purify GFPlva(XP), RBS+YFPlva+term(XP) | ||
| Line 418: | Line 378: | ||
[[image:3167.jpg]] | [[image:3167.jpg]] | ||
| - | |||
4.Ligate RBS with CFPlva+term | 4.Ligate RBS with CFPlva+term | ||
| Line 440: | Line 399: | ||
[[image:3169.jpg]] | [[image:3169.jpg]] | ||
| - | |||
4.Purify RBS+CFPlva+term(XP), RBS(SP) | 4.Purify RBS+CFPlva+term(XP), RBS(SP) | ||
| Line 470: | Line 428: | ||
[[image:3170.jpg]] | [[image:3170.jpg]] | ||
[[image:3171.jpg]] | [[image:3171.jpg]] | ||
| - | |||
| - | |||
5.Ligate pLac with RBS+GFPlva+term, pLac wtih RBS+RFPlva+term, pLac with RBS+YFPlva+term, pLac with RBS+CFPlva+term | 5.Ligate pLac with RBS+GFPlva+term, pLac wtih RBS+RFPlva+term, pLac with RBS+YFPlva+term, pLac with RBS+CFPlva+term | ||
| Line 480: | Line 436: | ||
2.Run gel for pLac+RBS+GFPlva+term, pLac+RBS+RFPlva+term, pLac+RBS+YFPlva+term, pLac+RBS+CFPlva+term | 2.Run gel for pLac+RBS+GFPlva+term, pLac+RBS+RFPlva+term, pLac+RBS+YFPlva+term, pLac+RBS+CFPlva+term | ||
| + | [[image:3172.jpg]] | ||
| Line 488: | Line 445: | ||
3.Run gel for pLac+RBS+GFPlva+term(XP), pLac+RBS+RFPlva+term(XP), pLac+RBS+YFPlva+term(XP), pLac+RBS+CFPlva+term(XP) | 3.Run gel for pLac+RBS+GFPlva+term(XP), pLac+RBS+RFPlva+term(XP), pLac+RBS+YFPlva+term(XP), pLac+RBS+CFPlva+term(XP) | ||
| + | |||
| + | [[image:3173.jpg]] | ||
4.Purify pLac+RBS+GFPlva+term(XP), pLac+RBS+RFPlva+term(XP), pLac+RBS+YFPlva+term(XP), pLac+RBS+CFPlva+term(XP) | 4.Purify pLac+RBS+GFPlva+term(XP), pLac+RBS+RFPlva+term(XP), pLac+RBS+YFPlva+term(XP), pLac+RBS+CFPlva+term(XP) | ||
| Line 498: | Line 457: | ||
pLac+RBS+CFPlva+term(XP)1 21.79 ng/uL | pLac+RBS+CFPlva+term(XP)1 21.79 ng/uL | ||
pLac+RBS+CFPlva+term(XP)2 7.87 ng/uL | pLac+RBS+CFPlva+term(XP)2 7.87 ng/uL | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
==2010-09-11== | ==2010-09-11== | ||
| Line 516: | Line 467: | ||
4.After the gel result appeared ,run the gel for YFP and CFP which had been purified to extract the correct length.(load 20ul) | 4.After the gel result appeared ,run the gel for YFP and CFP which had been purified to extract the correct length.(load 20ul) | ||
| - | + | [[image:3174.jpg]] | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
{|border="1" | {|border="1" | ||
|- | |- | ||
| Line 543: | Line 485: | ||
|} | |} | ||
Extract the gel and purify them. | Extract the gel and purify them. | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
Extract pLac+GFPlva ,pLac+RBS+YFPlva+Term and pLac+RBS+CFPlva+Term. | Extract pLac+GFPlva ,pLac+RBS+YFPlva+Term and pLac+RBS+CFPlva+Term. | ||
| Line 575: | Line 507: | ||
==2010-09-30== | ==2010-09-30== | ||
1.Run the gel for sspB, the front part of RBS+CFP+term, the front part of RBS+YFP+term, the front part of RBS+RFPlva+term, the back part of RBS+CFP+term, the back part of RBS+YFP+term, the back part of RFPlva and RFP | 1.Run the gel for sspB, the front part of RBS+CFP+term, the front part of RBS+YFP+term, the front part of RBS+RFPlva+term, the back part of RBS+CFP+term, the back part of RBS+YFP+term, the back part of RFPlva and RFP | ||
| + | |||
| + | [[image:3176.jpg]] | ||
2.Purify sspB, the front part of RBS+CFP+term, the front part of RBS+YFP+term, the front part of RBS+RFPlva+term, the back part of RBS+CFP+term, the back part of RBS+YFP+term and RFP | 2.Purify sspB, the front part of RBS+CFP+term, the front part of RBS+YFP+term, the front part of RBS+RFPlva+term, the back part of RBS+CFP+term, the back part of RBS+YFP+term and RFP | ||
| Line 596: | Line 530: | ||
2.Run the gel for pLac+RFP | 2.Run the gel for pLac+RFP | ||
| + | |||
| + | [[image:3177.jpg]] | ||
| + | |||
== 2010-10-7== | == 2010-10-7== | ||
| Line 627: | Line 564: | ||
7.Run gel for pLac+RBS+YFP+term and pLac+RBS+CFP+term(PCR) | 7.Run gel for pLac+RBS+YFP+term and pLac+RBS+CFP+term(PCR) | ||
| + | |||
| + | [[image:3179.jpg]] | ||
8.Digest pLac+BRS+RFP+term(XP)and pSB1C3+RFP(XP) | 8.Digest pLac+BRS+RFP+term(XP)and pSB1C3+RFP(XP) | ||
| Line 641: | Line 580: | ||
2.Run the gel for checking pLac+RFPlva | 2.Run the gel for checking pLac+RFPlva | ||
| + | |||
| + | [[image:3182.jpg]] | ||
3.Run the Gel for pLac+RBS+YFP+Term and pLac+RBS+YFP+Term(PCR)(digestion(XP)) | 3.Run the Gel for pLac+RBS+YFP+Term and pLac+RBS+YFP+Term(PCR)(digestion(XP)) | ||
| + | |||
| + | [[image:3181.jpg]] | ||
4.Run the gel for pLac+RFP(XP)and RFP+pSB1C3(XP) | 4.Run the gel for pLac+RFP(XP)and RFP+pSB1C3(XP) | ||
| + | |||
| + | [[image:3183.jpg]] | ||
5.Extract GFP, K145205 and pLac | 5.Extract GFP, K145205 and pLac | ||
| Line 659: | Line 604: | ||
2.Run gel for pLac+RBS+YFP+term, pLac+RBS+CFP+term | 2.Run gel for pLac+RBS+YFP+term, pLac+RBS+CFP+term | ||
| + | |||
| + | [[image:3180.jpg]] | ||
| + | |||
== 2010-10-21== | == 2010-10-21== | ||
| Line 664: | Line 612: | ||
2.Run gel for pLac+RBS+GFP+term, pLac+RBS+YFP+term, pLac+RBS+CFP+term | 2.Run gel for pLac+RBS+GFP+term, pLac+RBS+YFP+term, pLac+RBS+CFP+term | ||
| + | |||
| + | [[image:3184.jpg]] | ||
| + | [[image:3186.jpg]] | ||
| + | |||
{{:Team:NYMU-Taipei/Footer}} | {{:Team:NYMU-Taipei/Footer}} | ||
Latest revision as of 18:08, 27 October 2010
| Home | Project Overview | Speedy reporter | Speedy switch | Speedy protein degrader | Experiments and Parts | Applications | F.A.Q | About Us |
Our Goal
GFP
Purpose
Insert lva tag to the GFP by using PCR and ligate to pLac.
Material
[http://partsregistry.org/Part:BBa_R0010 BBa_R0010]: pLac
[http://partsregistry.org/Part:BBa_E0240 BBa_E0240]: RBS([http://partsregistry.org/Part:BBa_B0034 BBa_B0034]) +GFP([http://partsregistry.org/Part:BBa_E0040 BBa_E0040]) +Terminator([http://partsregistry.org/Part:BBa_B0015 BBa_B0015])
Parts Length
| DNA Length | ||
| Part | Part Length | Length in pSB1A2 |
| E0240 | 876bp | 1114bp |
| E0240+LVA | 909bp | 1147bp |
| R0010+E0240+LVA | 1117bp | 1355bp |
RFP
Purpose
Insert lva tag to the RFP by using PCR and ligate to pLac.
Material
BBa_R0010:pLac
[http://partsregistry.org/Part:BBa_I13507 BBa_I13507]: RBS(BBa_B0034)+RFP([http://partsregistry.org/Part:BBa_E1010 BBa_E1010])+Terminator(BBa_B0015)
Parts Length
| DNA Length | ||
| Part | Part Length | Length in pSB1A2 |
| I13507 | 861bp | 1099bp |
| I13507+LVA | 894bp | 1132bp |
| R0010+I13507+LVA | 1102bp | 1340bp |
CFP
Purpose
1.After ligate CFPlva to Terminator and RBS , ligate to pLac.
2.Remove LVA from the sequences to construct the control.
Material
BBa_R0010: pLac
BBa_B0034: RBS
[http://partsregistry.org/Part:BBa_E0022 BBa_E0022]: CFPlva
BBa_B0015: Terminator
Parts Length
| DNA Length | ||
| Part | Part Length | Length in pSB1A2 |
| E0022 | 762bp | 1000bp |
| E0022+B0015 | 899bp | 1137bp |
| B0034+E0022+B0015 | 919bp | 1157bp |
| R0010+B0034+E0022+B0015 | 1127bp | 1365bp |
YFP
Purpose
1.After ligate YFPlva to Terminator and RBS , ligate to pLac.
2.Remove LVA from the sequences to construct the control.
Material
BBa_R0010: pLac
BBa_B0034: RBS
[http://partsregistry.org/Part:BBa_E0032 BBa_E0032]: YFPlva
BBa_B0015: Terminator
Parts Length
| DNA Length | ||
| Part | Part Length | Length in Plasmid |
| E0032 | 762bp | 1000bp |
| E0032+B0015 | 899bp | 1137bp |
| B0034+E0032+B0015 | 919bp | 1157bp |
| R0010+B0034+E0032+B0015 | 1127bp | 1365bp |
Our Experiment Data
2010-07-22
1. Extract B0034(RBS) from 2010 plate 1 2U
Extract B0015(Terminator) from 2010 plate 1 23L
2.Transform into 15ul competent cells.
3.Add 85ul LB after heat shock.
4.Culture for 30 minutes.
5.80ul for transformation ; 20ul for liquid culture
6.Incubate at 6:00pm.
2010-07-23
1.Extract RBS and Term(abbreviation for terminator) cultured in 7/22.
2.Measured the nano drop.
B0015:26.94ng/ul B0034:45.59ng/ul
3.Digest B0034(SP) and B0015(XP) at 11:30am
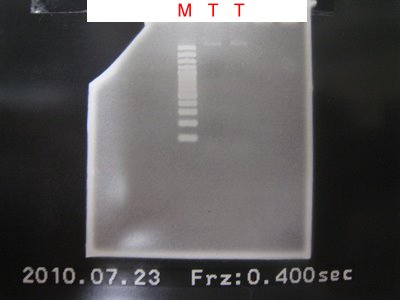
4.Run the gel for B0015.
5.Measured the nano drop.
B0034:3.85ng/ul
6.Purify B0034.
7.Ligate YFPlva and CFPlva to Term.
8.Transform into 30ul competent cells.
9.PCR to make GFPlva and RFPlva front parts.
2010-07-24
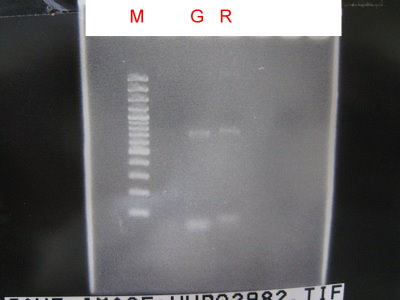
1.Do 3 in 1 and PCR to check CFPlva+Terminator ,YFPlva+Terminator ,RBS , Terminator.
2.Run the gel.
3.PCR to make the whole parts GFPlva and RFPlva.
4.Run the gel.
5.Digest GFPlva(XP) and RFPlva(XP).
6.Measure the nano drop.
GFPlva:8.66ng/ul RFPlva:7.35ng/ul
7.Ligate GFPlva and RFPlva to psB1A2.
8.Tranform into 30ul competent cells.
2010-07-26
1.Digest RBS(SP), CFP(XP), YFP(XP) and Term(P)
2.Run the gel for .
3.Digest CFPlva and YFPlva.
4.Ligate CFPlva and YFPlva to Term.
2010-07-28
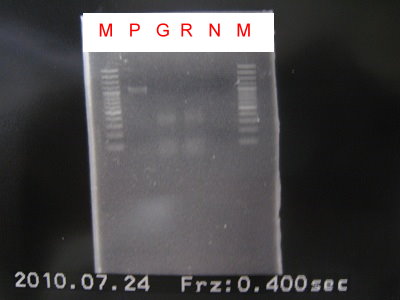
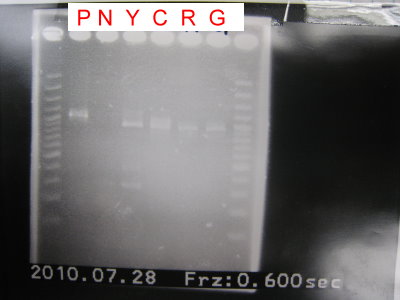
1.Do 3 in 1 to check GFPlva ,RFPlva , YFPlva+Term and CFPlva+Term.
2.Run the gel.
2010-07-29
1.Extract GFPlva and RFPlva cultured on 7/29.
2.Digest GFPlva(X,NdeI) and RFPlva(X,NcoI) for checking if they were correct. Digest YFPlva(E) ,CFPlva(E) and Term(E) for ligation.
3.After 10 minutes ,degrade EcoRI by rising the temperature to 80 for 5 minutes.
4.Run the gel for YFPlva ,CFPlva and Term and purify them.
5.Digest YFPlva(ES) ,CFPlva(ES) and Term(EX).
6.Measure the nano drop.
7.Run the gel for YFPlva(ES) ,CFPlva(ES) ,Term(EX) ,GFPlva and RFPlva.
8.Ligate CFPlva(ES) and YFPlva(ES) to Term(EX).
9.PCR the whole part for GFPlva and RFPlva again.
2010-07-31
1.Do 3 in 1 for YFPlva+Term and CFPlva+Term.
2.Run the gel for YFPlva+Term ,CFPlva+Term ,RFPlva and GFPlva.
3.Ligate YFPlva(ES) to Term(EX).
4.PCR the front part of GFPlva and RFPlva for checking the condition is right or wrong.
5.Run the gel to make the front part of GFPlva and RFPlva.
2010-08-02
1.Do 3 in 1 for YFPlva+Term.
2.Digaest CFPlva+Term ,RBS(SP) ,Term(ES) and Term(E).
3.Measure the nano drop.
4.PCR to make the front part of GFPlva and RFPlva.
5.Ligate RBS(SP) to CFPlva+Term (XP).
6.Liquid culture for RBS.
2010-08-04
1.PCR to make the whole part of GFPlva and RFPlva.
2.Extract GFPlva ,RFPlva ,YFPlva+Term ,RBS.
2010-08-05
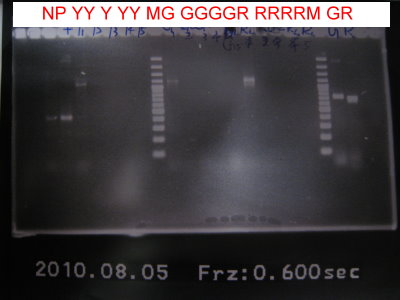
1.PCR for RFPlva, GFP without RBS, RFP without RBS, RBS+YFP+term, GFPlva.
2.Run gel for RBS+YFP+term, GFPlva, RFPlva, GFP without RBS, RFP without RBS
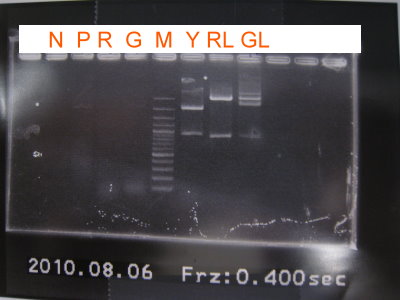
2010-08-06
1.PCR for front part of GFP without RBS and front part of RFP without RBS
2.Extraction for YFP, GFPlva, RFPlva
3.Digestion
4.Run gel for RBS+YFP+term
5.PCR again for GFP without RBS, RFP without RBS
6.Purification for YFP, GFPlva, RFPlva
7.Nanodrop
GFPlva(XP) 3.89ng/uL RFPlva(XP) 4.48ng/uL RBS+YFP+term 3.18ng/uL
8.Ligate CFP+term with RBS
9.Transformation
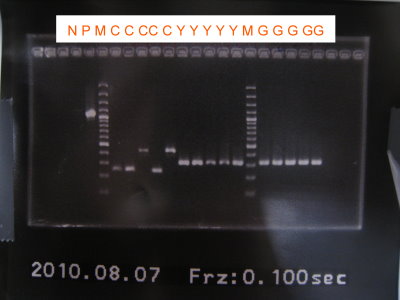
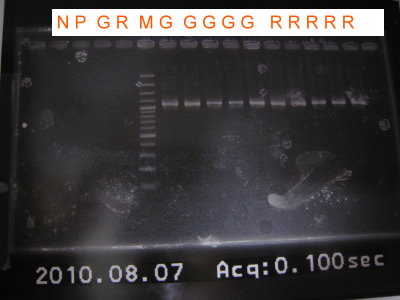
2010-08-07
1.Colony PCR for RBS+CFP+Term, GFP, RFP
2.Run gel for RBS+CFP+term, GFP, RFP
2010-08-09
1.Extraction for GFP, RFP
2.Colony PCR for GFP
2010-08-10
1.Digestion for GFPlva(XP), YFPlva(XP), RFPlva(XP), CFP+Term(XP)
2.Run gel for GFPlva(XP), YFPlva(XP), RFPlva(XP), CFP+Term(XP)
2010-08-11
1.Extract CFP+term
2.Real PCR for GFP wihout RBS, RFP without RBS
3.Ligate RBS with CPF+Term
4.Transform RBS with CPF+Term, pLac(R0010)
2010-08-12
1.Colony PCR for RBS+CFP+Term, pLac
2.Run gel for GFP without RBS, RFP without RBS, pLac
2010-08-14
1.Extract pLac
2.Digest pLac(SP)
3.Nanodrop
pLac1 5.73ng/uL pLac2 2.70ng/uL pLac3 4.08ng/uL
2010-08-17
1.Ligate RBS with CFP+term
2.Transform RBS with CFP+term
3.Digest GFPlva(XP), RBS+YFPlva+term(XP)
4.Run gel for GFPlva(XP), RBS+YFPlva+term(XP)
5.Purify GFPlva(XP), RBS+YFPlva+term(XP)
6.Nanodrop
GFPlva(XP) 8.87 ng/uL RBS+YFPlva+term(XP) 5.01 ng/uL
7.Culture GFPlva, RBS+YFPlva+term
2010-08-18
1.Extract GFPlva, RBS+YFPlva+term
2.Digest RBS, CFP+term
3.Ligate CFP+term with RBS
2010-08-19
1.Colony PCR for RBS+CFPlva+Term
2.Nanodrop
RBS(SP) 21.38ng/uL CFP+Term(XP) 10.38ng/uL
3.Run gel for RBS+CFPlva+Term
4.Ligate RBS with CFPlva+term
5.Transform
2010-08-20
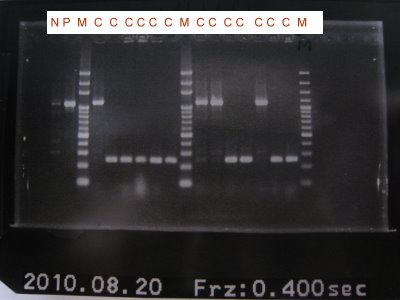
1.Colony PCR for RBS+CFPlva+term
2.Run gel for RBS+CFPlva+term
2010-08-21
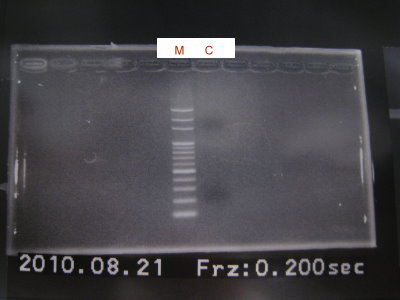
1.Extract for RFP+CFPlva+term
2.Digest RBS+CFPlva+term(XP), RBS(SP)
3.Run gel for RBS+CFPlva+term
4.Purify RBS+CFPlva+term(XP), RBS(SP)
5.Nanodrop
RBS1 2.96 ng/uL RBS2 2.02 ng/uL RBS+CFPlva+term 8.98 ng/uL
2010-08-23
1.Ligate pLac with RBS+GFPlva+term, pLac with RBS+YFPlva+term, pLac with RBS+RFPlva+term
2.Transform pLac with RBS+GFPlva+term, pLac with RBS+YFPlva+term, pLac with RBS+RFPlva+term
2010-08-24
1.Colony PCR for pLac+RBS+GFPlva+term, pLac+RBS+YFPlva+term, pLac+RBS+RFPlva+term
2010-09-01
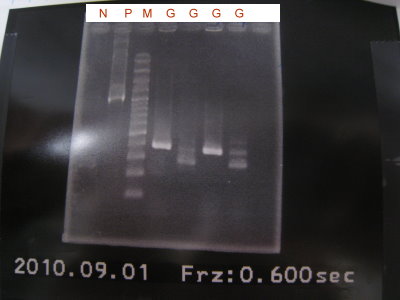
1.Colony PCR for pLac+RBS+GFPlva+term
2.Extract RBS+CFPlva+term
3.Digest RBS+CFPlva+term(XP)
4.Run the gel for pLac+RBS+GFPlva+term,RBS+CFPlva+term(XP)
5.Ligate pLac with RBS+GFPlva+term, pLac wtih RBS+RFPlva+term, pLac with RBS+YFPlva+term, pLac with RBS+CFPlva+term
2010-09-02
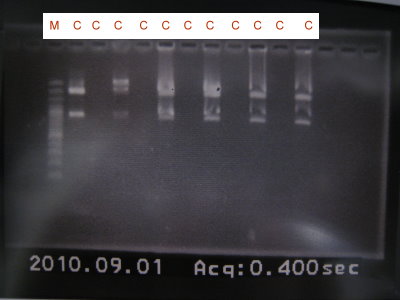
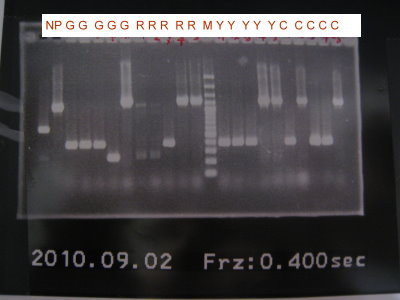
1.Colony PCR for pLac+RBS+GFPlva+term, pLac+RBS+RFPlva+term, pLac+RBS+YFPlva+term, pLac+RBS+CFPlva+term
2.Run gel for pLac+RBS+GFPlva+term, pLac+RBS+RFPlva+term, pLac+RBS+YFPlva+term, pLac+RBS+CFPlva+term
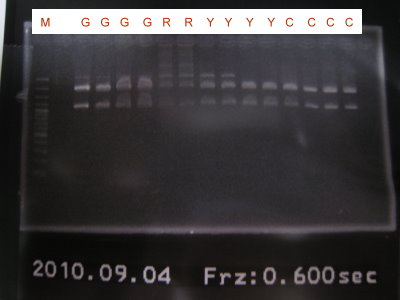
2010-09-04
1.Extract pLac+RBS+GFPlva+term, pLac+RBS+RFPlva+term, pLac+RBS+YFPlva+term, pLac+RBS+CFPlva+term
2.Digest pLac+RBS+GFPlva+term(XP), pLac+RBS+RFPlva+term(XP), pLac+RBS+YFPlva+term(XP), pLac+RBS+CFPlva+term(XP)
3.Run gel for pLac+RBS+GFPlva+term(XP), pLac+RBS+RFPlva+term(XP), pLac+RBS+YFPlva+term(XP), pLac+RBS+CFPlva+term(XP)
4.Purify pLac+RBS+GFPlva+term(XP), pLac+RBS+RFPlva+term(XP), pLac+RBS+YFPlva+term(XP), pLac+RBS+CFPlva+term(XP)
5.Nanodrop
pLac+RBS+GFPlva+term(XP)1 22.31 ng/uL pLac+RBS+GFPlva+term(XP)2 14.16 ng/uL pLac+RBS+RFPlva+term(XP) 8.6 ng/uL pLac+RBS+YFPlva+term(XP) 13.52 ng/uL pLac+RBS+CFPlva+term(XP)1 21.79 ng/uL pLac+RBS+CFPlva+term(XP)2 7.87 ng/uL
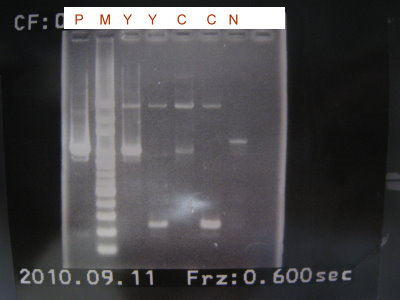
2010-09-11
1.PCR to make the front part and back part of the YFP and CFP. (in order to remove lva tag)
2.Run the gel for the front part and back part of YFP and CFP.(load 5ul)
3.While running the gel ,we purified them.
4.After the gel result appeared ,run the gel for YFP and CFP which had been purified to extract the correct length.(load 20ul)
| template | forward primer | reverse primer | |
| positive | RFP | VF2 | VR |
| YFP front part | pLac+RBS+YFPlva+Term | VF2 | XFP lva remover rp |
| YFP back part | pLac+RBS+YFPlva+Term | XFP lva remover fp | VR |
| CFP front part | pLac+RBS+CFPlva+Term | VF2 | XFP lva remover rp |
| CFP back part | pLac+RBS+CFPlva+Term | XFP lva remover fp | VR |
Extract the gel and purify them.
Extract pLac+GFPlva ,pLac+RBS+YFPlva+Term and pLac+RBS+CFPlva+Term.
Measure the nano drop.
pLac+GFPlva: 170.51ng/ul
pLac+RBS+YFPlva+Term: 276.08ng/ul
pLac+RBS+YFPlva+Term: 287.46ng/ul
pLac+RBS+CFPlva+Term: 116.21ng/ul
pLac+RBS+CFPlva+Term: 252.12ng/ul
2010-09-18
1.Extract RFP.
2.Do the PCR to make the front part and back part of the RFPlva and to make the whole part of pLac+RBS+YFP+Term and pLac+RBS+YFP+Term.
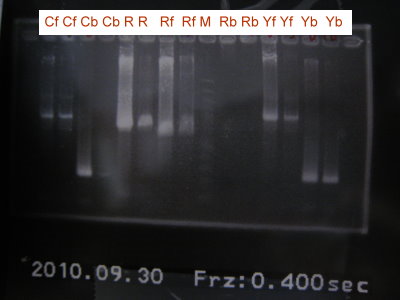
2010-09-30
1.Run the gel for sspB, the front part of RBS+CFP+term, the front part of RBS+YFP+term, the front part of RBS+RFPlva+term, the back part of RBS+CFP+term, the back part of RBS+YFP+term, the back part of RFPlva and RFP
2.Purify sspB, the front part of RBS+CFP+term, the front part of RBS+YFP+term, the front part of RBS+RFPlva+term, the back part of RBS+CFP+term, the back part of RBS+YFP+term and RFP
3.Measure nanodrop
sspB 34.73ng/uL the front part of RBS+CFP+term 15.82ng/uL the front part of RBS+YFP+term 35.53ng/uL the front part of RBS+RFPlva+term 76.10ng/uL the back part of RBS+CFP+term 34.89ng/uL the back part of RBS+YFP+term 36.27ng/uL RFP 79.82ng/uL
2010-10-1
1.PCR to make back part of RFPlva
2.Digest pLac(SP), sspB(PCR) and RFP(PCR)
2010-10-4
1.3-in-1 for pLac+RFP
2.Run the gel for pLac+RFP
2010-10-7
1.Extract RFPlva(PCR)
RFPlva 100ng/uL
2.Digest RFPlva(XP)
2010-10-9
1.3-in-1 for K145205
2.PCR to make the whole part of pLac+RBS+YFP+term and pLac+RBS+CFP+term
3.Purify RFP(PCR)(XP)
Nanodrop: RFP 42.3ng/uL
4.Ligate pLac(SP)+RBS+RFP+term(PCR)(XP)
5.Run gel for checking K145205
6.Purify pLac+BRS+RFP+term and pSB1C3+RFP
Nanodrop: pLac+RFP1 72.8ng/uL pLac+RFP2 22.5ng/uL pSB1C3+RFP1 105.4ng/uL pSB1C3+RFP2 91.5ng/uL pSB1C3+RFP3 65.3ng/uL
7.Run gel for pLac+RBS+YFP+term and pLac+RBS+CFP+term(PCR)
8.Digest pLac+BRS+RFP+term(XP)and pSB1C3+RFP(XP)
9.Extract pLac+BRS+RFP+term(XP)and pSB1C3+RFP(XP) and purify them
2010-10-11
1.Digest pLac+RBS+CFP+term(XP) and pLac+RBS+YFP+term(XP)
2.Ligate pLac+RFPlva
2010-10-12
1.3-in-1 for pLac+RFPlva
2.Run the gel for checking pLac+RFPlva
3.Run the Gel for pLac+RBS+YFP+Term and pLac+RBS+YFP+Term(PCR)(digestion(XP))
4.Run the gel for pLac+RFP(XP)and RFP+pSB1C3(XP)
5.Extract GFP, K145205 and pLac
6.Digest GFP(XP) and pLac(SP)
7.Purify pSB1A2(XP), pSB1C3(XP), pLac+RBS+RFP+term(XP), pLac+RBS+YFP+term(XP)and pLac+RBS+CFP+term(XP)
2010-10-14
1.Ligation for pLac+GFPlva, pLac+CFPlva, pLac+RFPlva, pLac+RBS+YFP+term with pSB1A2
2010-10-15
1.Colony PCR for pLac+RBS+YFP+term, pLac+RBS+CFP+term
2.Run gel for pLac+RBS+YFP+term, pLac+RBS+CFP+term
2010-10-21
1.Colony PCR for pLac+RBS+GFP+term, pLac+RBS+YFP+term, pLac+RBS+CFP+term
2.Run gel for pLac+RBS+GFP+term, pLac+RBS+YFP+term, pLac+RBS+CFP+term
 "
"