Team:NYMU-Taipei/Experiments/Riboswitch
From 2010.igem.org
(Difference between revisions)
(→2010.08.18) |
(→2010.10.22) |
||
| (110 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
{{:Team:NYMU-Taipei/Header}} | {{:Team:NYMU-Taipei/Header}} | ||
| + | =Parts= | ||
| + | *Ribo = Theophylline riboswitch([http://partsregistry.org/Part:BBa_K411001 BBa_K411001]) | ||
| + | *RV = Theophylline riboswitch([http://partsregistry.org/Part:BBa_K411001 BBa_K411001]) + pSB1A2 | ||
| + | *FRV = Theophylline riboswitch + GFP([http://partsregistry.org/Part:BBa_J04630 BBa_J04630]) + pSB1A2 | ||
| + | *PFRV = Theophylline riboswitch + GFP([http://partsregistry.org/Part:BBa_J04630 BBa_J04630]) + pLac([http://partsregistry.org/Part:BBa_R0010 BBa_R0010]) + pSB1A2 | ||
| + | |||
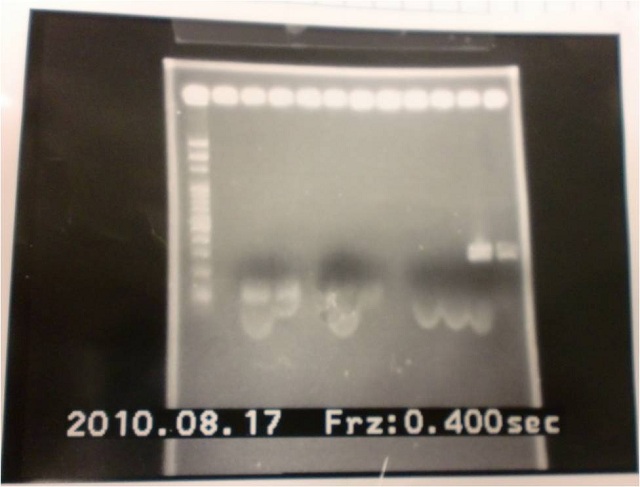
=2010.08.17 = | =2010.08.17 = | ||
*PCR of primer&Digestion&Ligation | *PCR of primer&Digestion&Ligation | ||
| Line 8: | Line 14: | ||
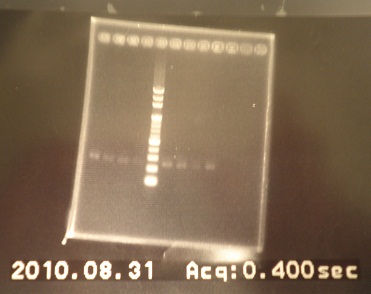
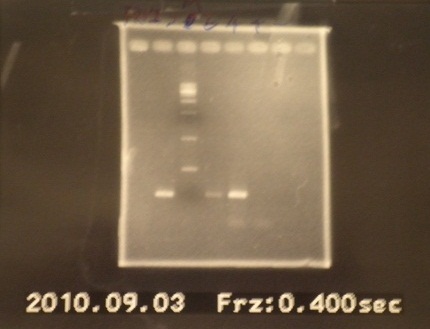
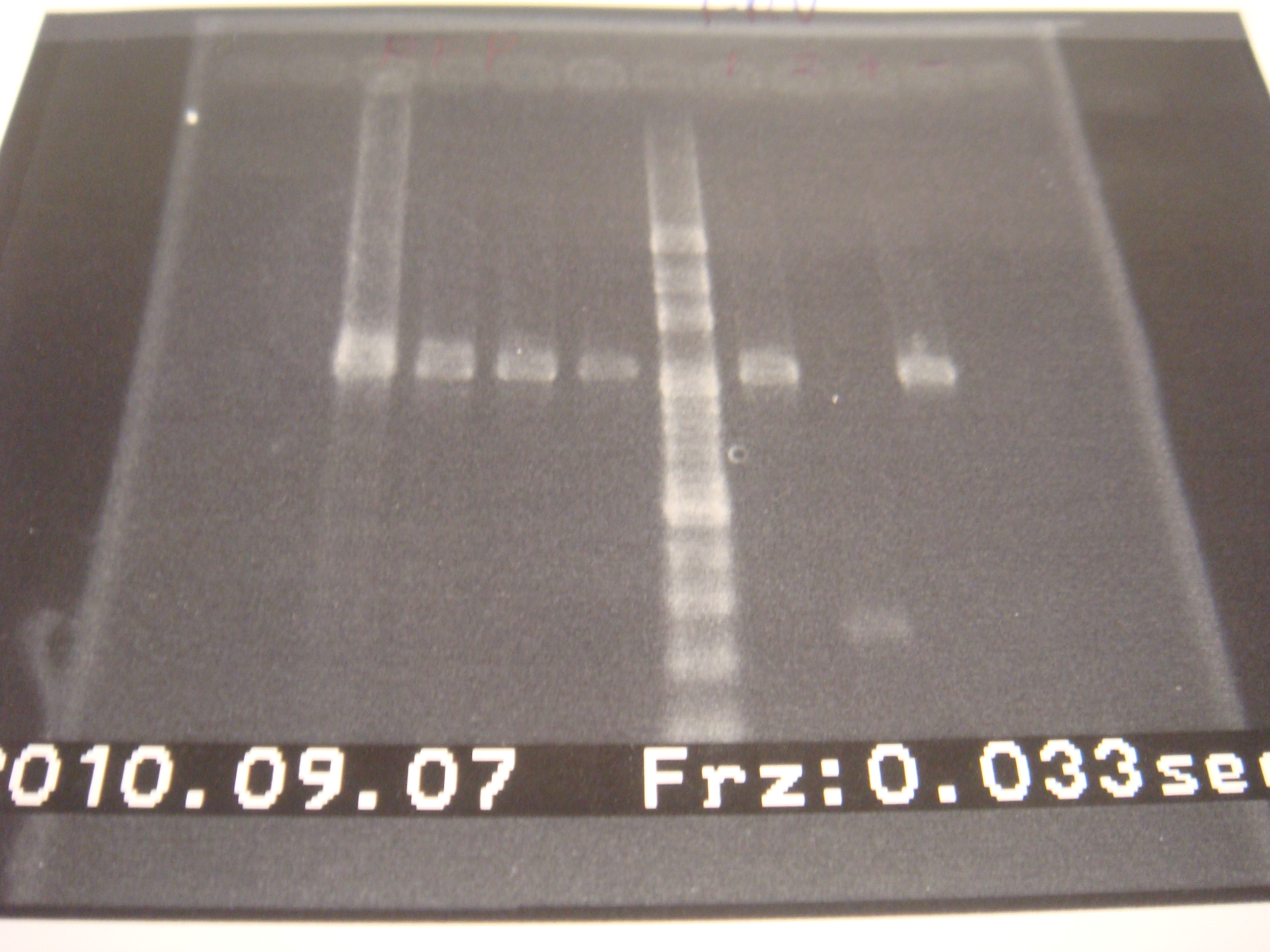
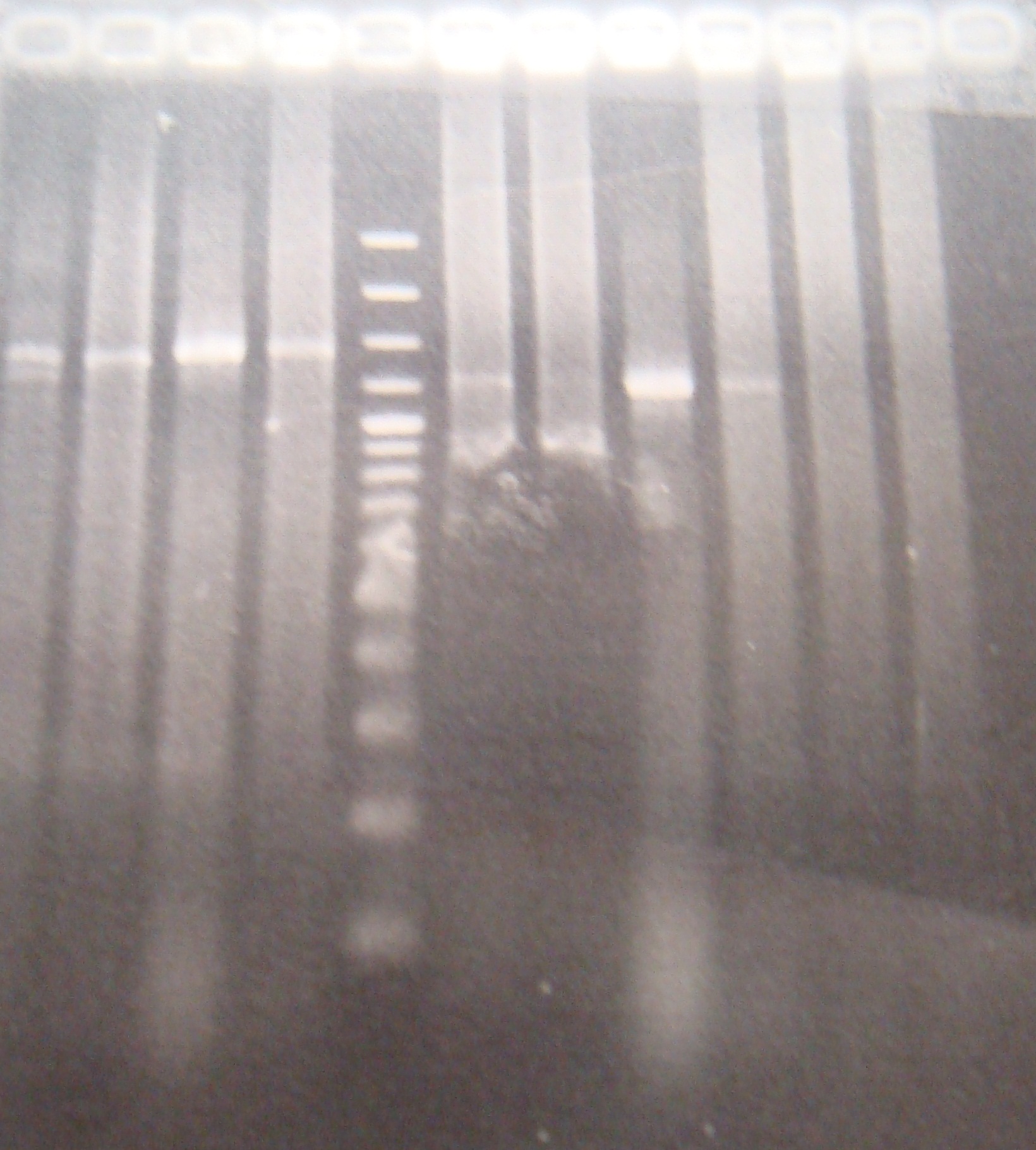
|1: marker 100bp|||n|= | |1: marker 100bp|||n|= | ||
|2: -|-|-|n|= | |2: -|-|-|n|= | ||
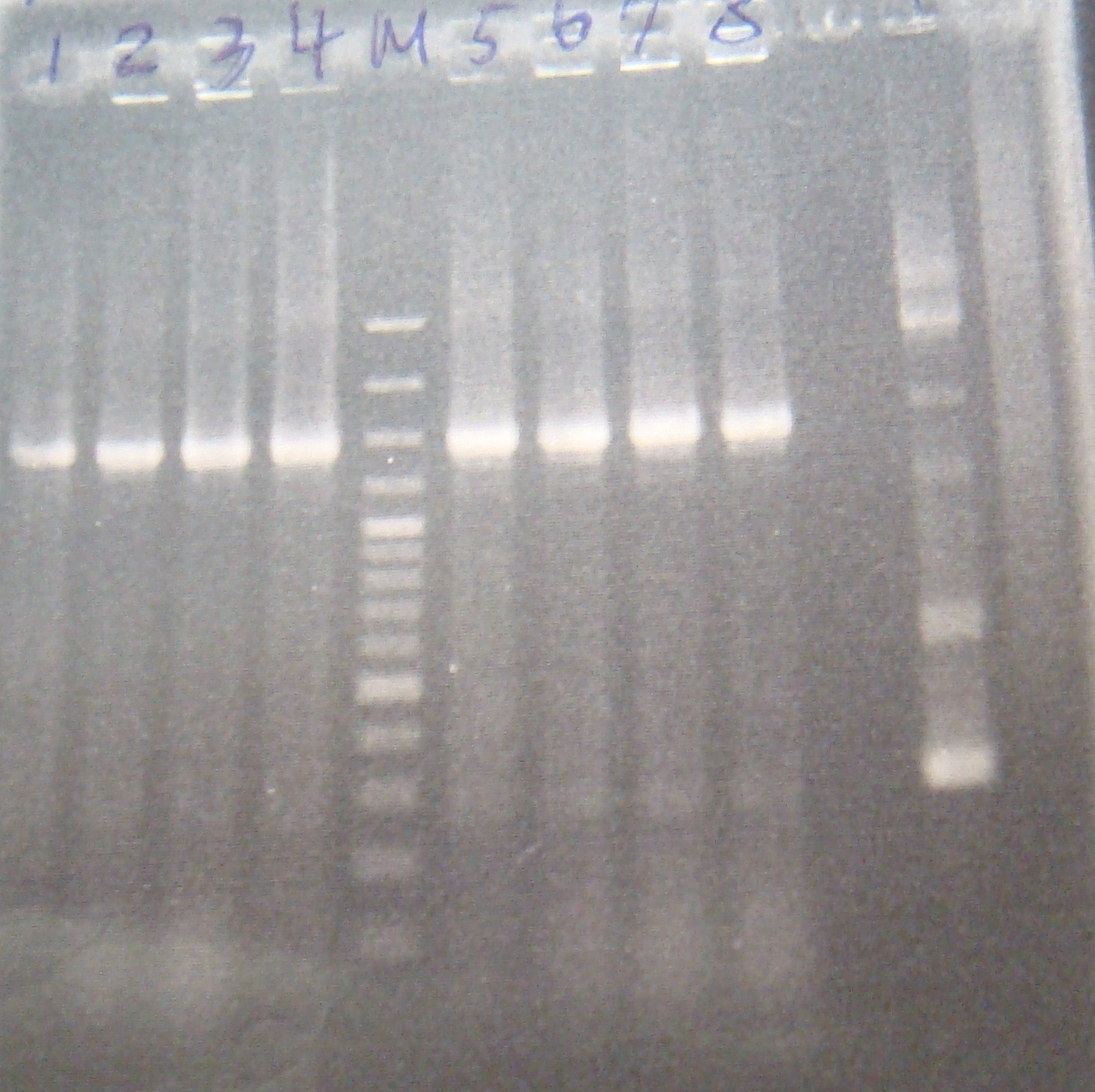
| - | |3: riboswitch |56 bp|none| | + | |3: riboswitch |56 bp|none|f|= |
| - | |4: riboswitch |56 bp|none| | + | |4: riboswitch |56 bp|none|f|= |
|5: -|-|-|n|= | |5: -|-|-|n|= | ||
| - | |6: riboswitch |56 bp|none| | + | |6: riboswitch |56 bp|none|f|= |
| - | |7: riboswitch |56 bp|none| | + | |7: riboswitch |56 bp|none|f|= |
|8: -|-|-|n|= | |8: -|-|-|n|= | ||
| - | |9: riboswitch |56 bp|none| | + | |9: riboswitch |56 bp|none|f|= |
| - | |10: riboswitch |56 bp|none| | + | |10: riboswitch |56 bp|none|f|= |
| - | |11: Positive Control|1100 bp|none| | + | |11: Positive Control|1100 bp|none|w|= |
| - | |12: Negative Control||Contamination ~300bp | | + | |12: Negative Control||Contamination ~300bp |w|= |
|}} | |}} | ||
{| border=1 | {| border=1 | ||
| Line 59: | Line 65: | ||
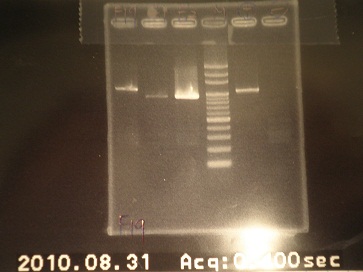
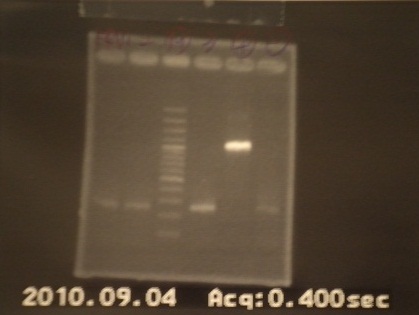
|4: -|-|-|n|= | |4: -|-|-|n|= | ||
|5: Positive Control|1100 bp|none|w|= | |5: Positive Control|1100 bp|none|w|= | ||
| - | |6: Negative Control||Contamination ~3000bp| | + | |6: Negative Control||Contamination ~3000bp|w|= |
|}} | |}} | ||
{| border=1 | {| border=1 | ||
| Line 108: | Line 114: | ||
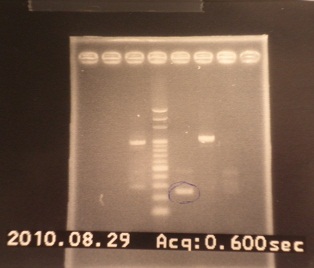
{{:Team:NYMU-Taipei/GELC|= | {{:Team:NYMU-Taipei/GELC|= | ||
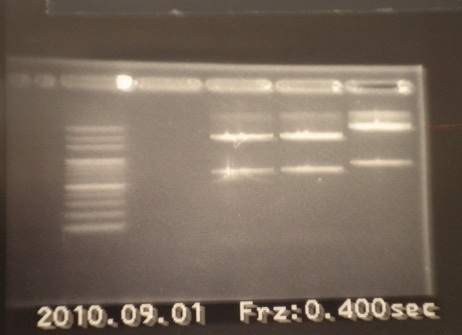
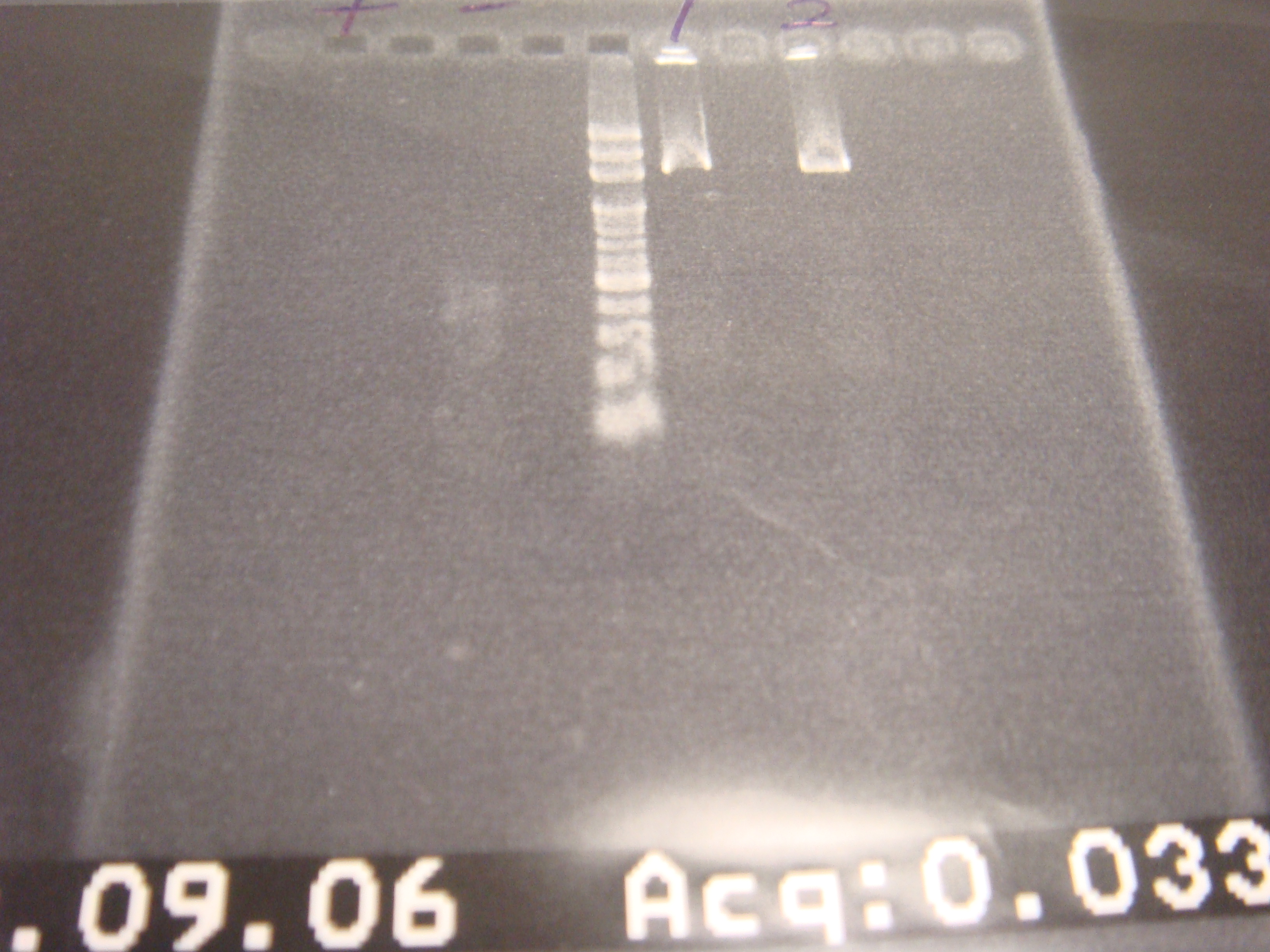
|1: marker 100bp|||n|= | |1: marker 100bp|||n|= | ||
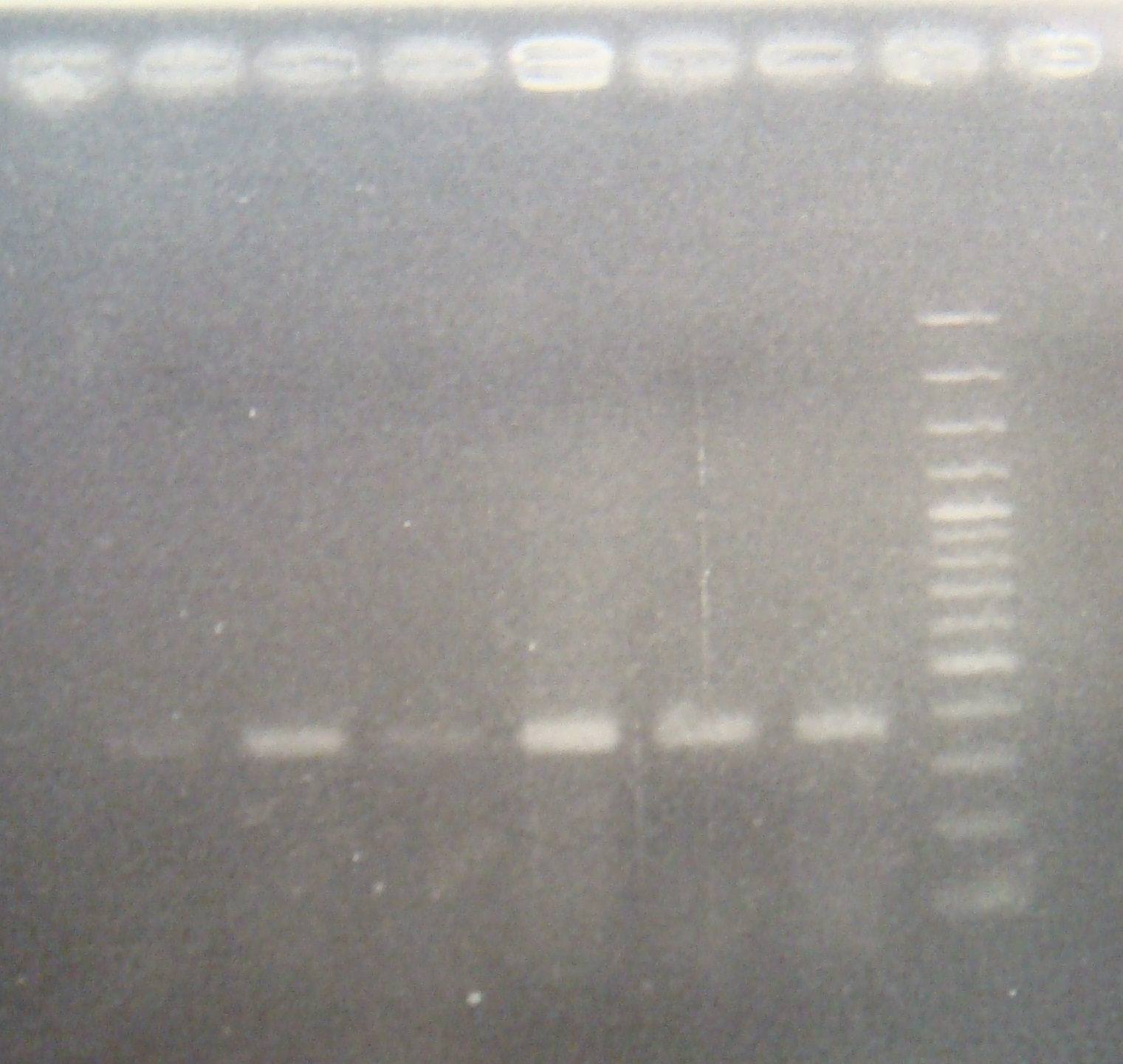
| - | |2: ribo+vector| | + | |2: ribo+vector|294bp||f|= |
| - | |3: ribo+vector| | + | |3: ribo+vector|294bp||f|= |
|4: -|-|-|n|= | |4: -|-|-|n|= | ||
|5: Positive Control|1100 bp|none|w|= | |5: Positive Control|1100 bp|none|w|= | ||
| - | |6: Negative Control||| | + | |6: Negative Control|||w|= |
|}} | |}} | ||
{| border=1 | {| border=1 | ||
| Line 134: | Line 140: | ||
=2010.08.23= | =2010.08.23= | ||
| - | *the upper experiment are failed,so we restart the experiment. | + | *the upper experiment are failed, so we restart the experiment. |
**Run gel 11:00 | **Run gel 11:00 | ||
**After nanodrop, we found that the concentration is too low(only 3.0), so we re-PCR-of-primer again. | **After nanodrop, we found that the concentration is too low(only 3.0), so we re-PCR-of-primer again. | ||
| Line 153: | Line 159: | ||
|2: marker 100bp|||n|= | |2: marker 100bp|||n|= | ||
|3: |||n|= | |3: |||n|= | ||
| - | |4: pcr riboswitch|56bp|| | + | |4: pcr riboswitch|56bp||f|= |
|5: |||n|= | |5: |||n|= | ||
| - | |6: Positive Control||300bp| | + | |6: Positive Control||300bp|w|= |
|7: Negative Control|||w|= | |7: Negative Control|||w|= | ||
|}} | |}} | ||
| Line 248: | Line 254: | ||
![[Image:NYMU R26-2.JPG]] !! | ![[Image:NYMU R26-2.JPG]] !! | ||
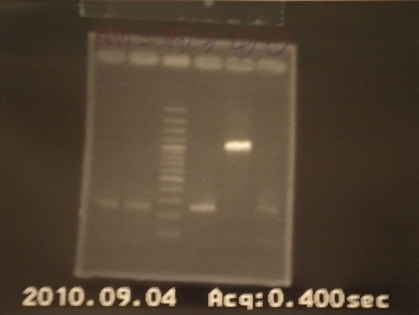
{{:Team:NYMU-Taipei/GELC|= | {{:Team:NYMU-Taipei/GELC|= | ||
| - | |1: GFP|||f|= | + | |1: GFP|720bp||f|= |
| - | |2: (1)riboswitch+pSB1A2| | + | |2: (1)riboswitch+pSB1A2|294bp||f|= |
| - | |3: (2)riboswitch+pSB1A2| | + | |3: (2)riboswitch+pSB1A2|294bp||f|= |
| - | |4: Positive Control|1100bp|| | + | |4: Positive Control|1100bp||w|= |
| - | |5: Negative Control|Contamination~1000 bp|| | + | |5: Negative Control|Contamination~1000 bp||w|= |
|6: Marker:100bp|||n|= | |6: Marker:100bp|||n|= | ||
|}} | |}} | ||
| Line 351: | Line 357: | ||
![[Image:NYMU R27.JPG]] !! | ![[Image:NYMU R27.JPG]] !! | ||
{{:Team:NYMU-Taipei/GELC|= | {{:Team:NYMU-Taipei/GELC|= | ||
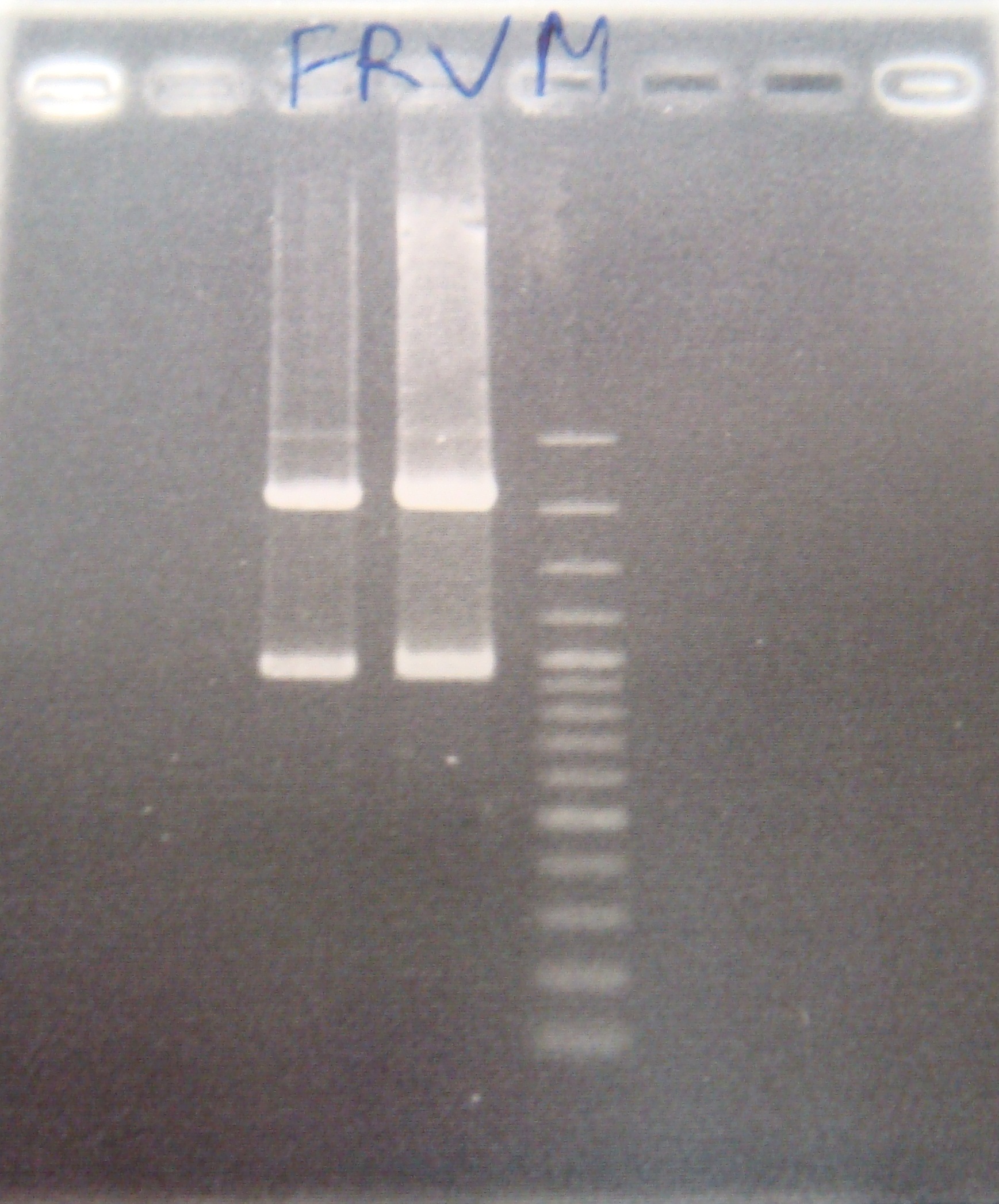
| - | |1: | | + | |1:FRV |1158bp|294bp|f|= |
| - | |2: | + | |2: FRV|1158bp|294bp|f|= |
| - | |3: | + | |3: 100bp marker|-|-|n|= |
| - | |4: | | + | |4: FRV|1158bp|294bp|f|= |
| - | |5: | + | |
| - | |6 | + | |5: positive control|1100bp| |w|= |
| - | + | |6: Negative Control|-|Contamination ~300bp|w|= | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
|}} | |}} | ||
{| border=1 | {| border=1 | ||
| Line 395: | Line 394: | ||
![[Image:NYMU P9010273.jpg]] !! | ![[Image:NYMU P9010273.jpg]] !! | ||
{{:Team:NYMU-Taipei/GELC|= | {{:Team:NYMU-Taipei/GELC|= | ||
| - | |1: | + | |1: FRV|1158bp|294bp|f|= |
| - | |2: | + | |2: FRV|1158bp|294bp|f|= |
| - | |3: | + | |3: FRV|1158bp|294bp|f|= |
|4: positive control|1100bp|-|w|= | |4: positive control|1100bp|-|w|= | ||
|5: marker100 bp|-|-|n|= | |5: marker100 bp|-|-|n|= | ||
| - | |6: negative control|-|-| | + | |6: negative control|-|-|w|= |
|}} | |}} | ||
| Line 436: | Line 435: | ||
|1: -|-|-|n|= | |1: -|-|-|n|= | ||
|2: -|-|-|n|= | |2: -|-|-|n|= | ||
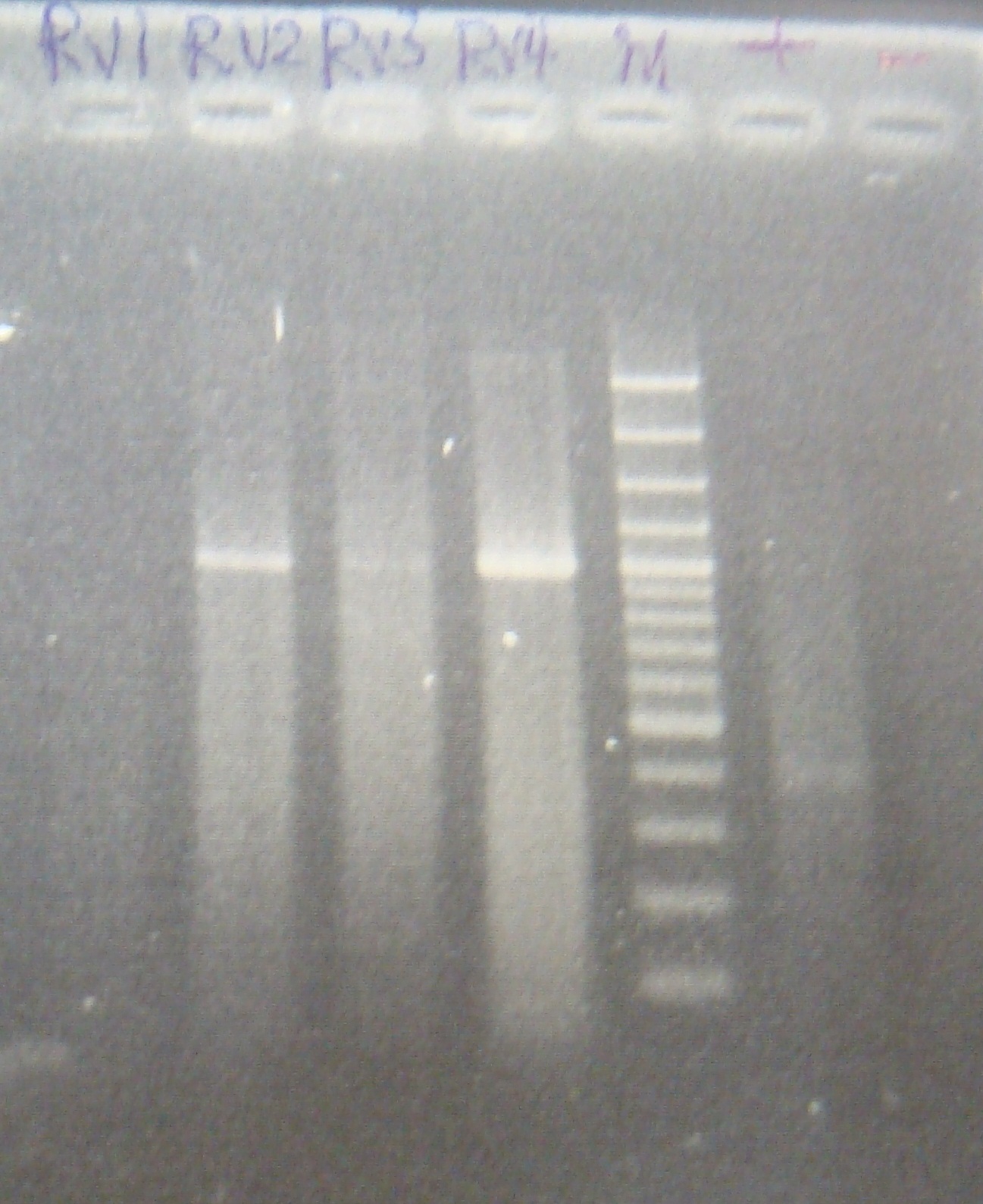
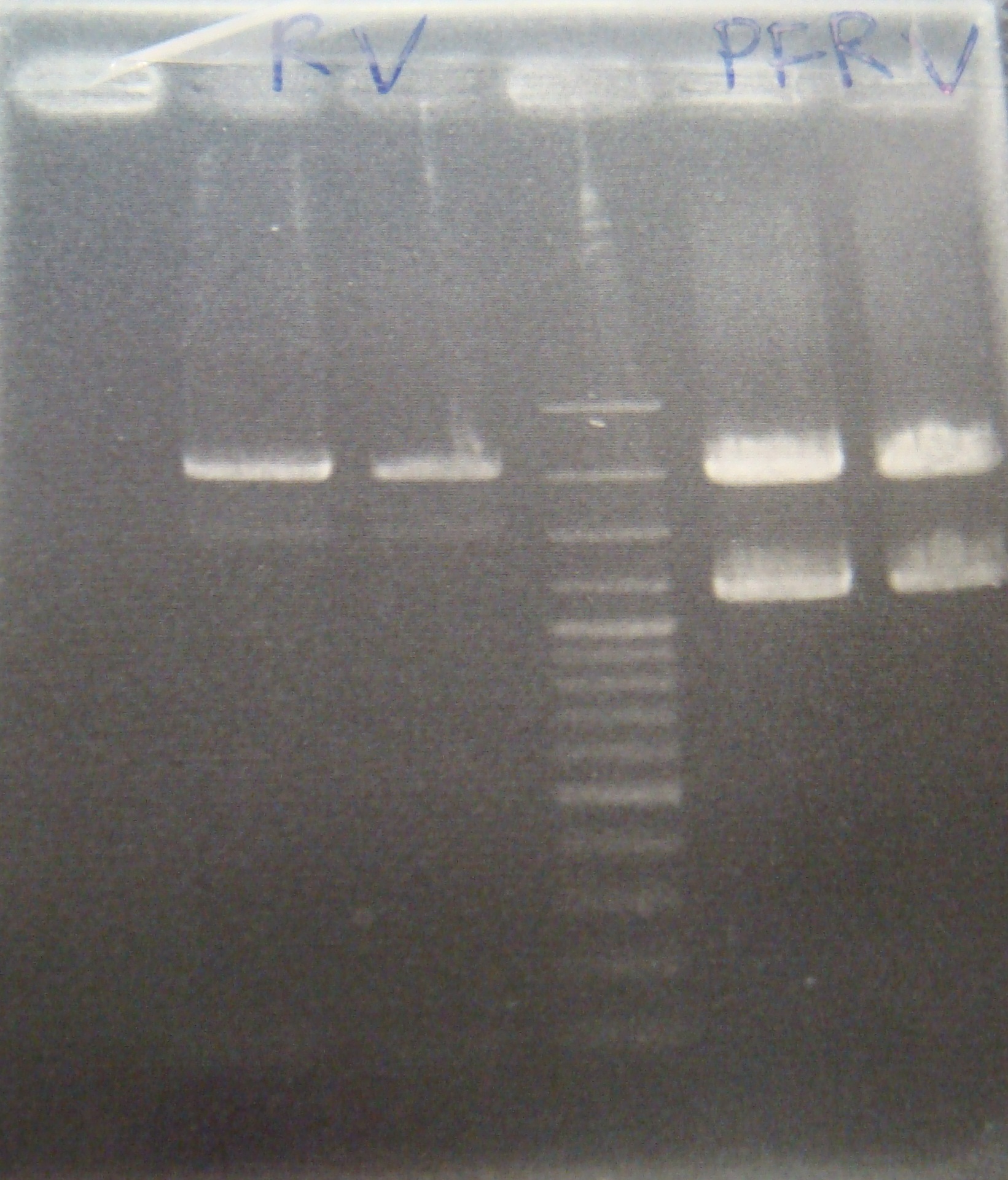
| - | |3: | + | |3: RV1|294bp|1100bp|f|= |
|4: marker100 bp|-|-|n|= | |4: marker100 bp|-|-|n|= | ||
| - | |5: | + | |5: RV2|294bp|-|f|= |
| - | |6: positive control|1100bp|-| | + | |6: positive control|1100bp|-|w|= |
| - | |7: negative control|-|-| | + | |7: negative control|-|-|w|= |
|}} | |}} | ||
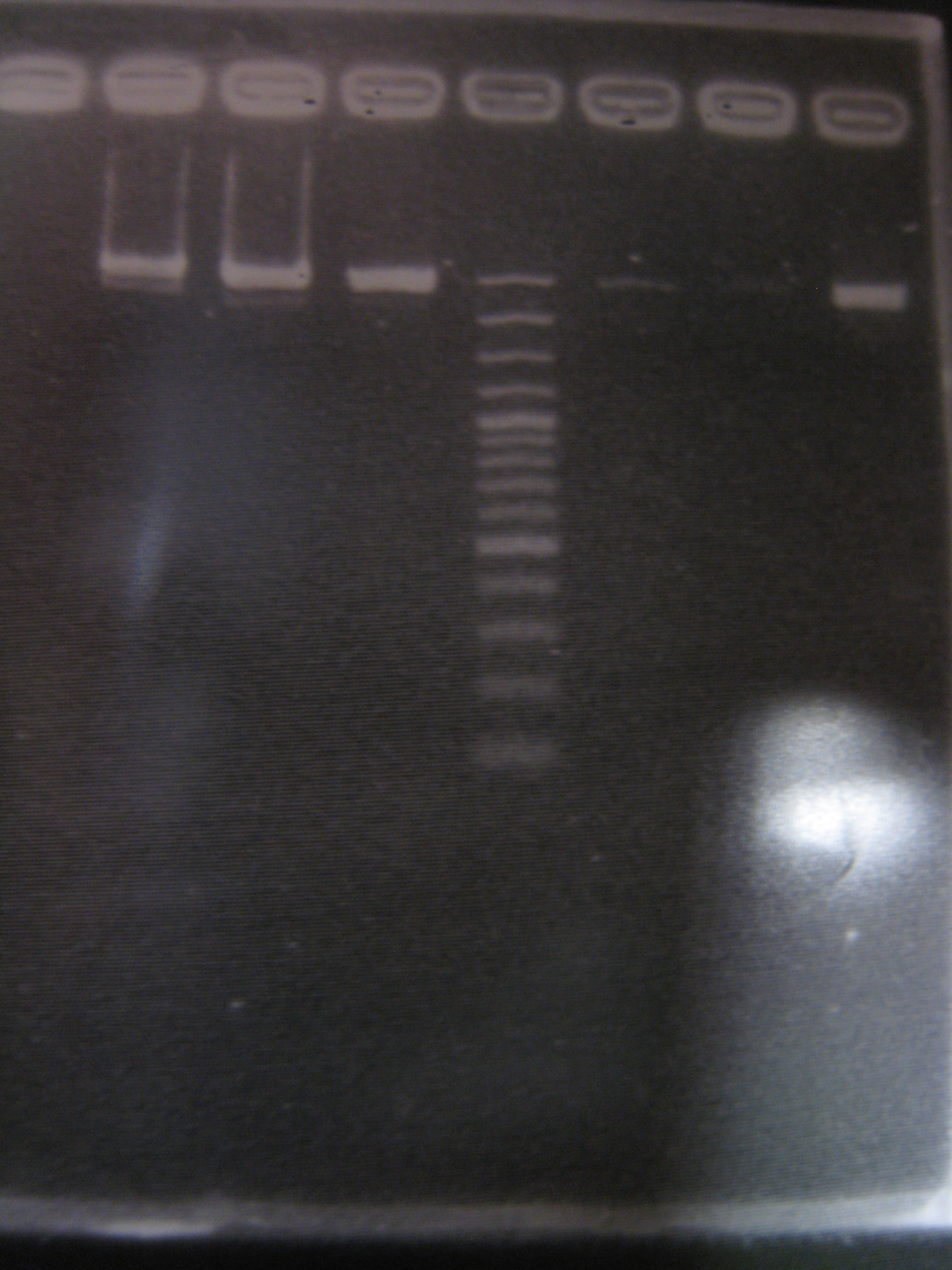
| Line 474: | Line 473: | ||
|3: GFP3|854bp||f|= | |3: GFP3|854bp||f|= | ||
|4: marker100 bp|-|-|n|= | |4: marker100 bp|-|-|n|= | ||
| - | |5: riboswitch+vector| | + | |5: riboswitch+vector|294bp|-|f|= |
| - | |6: negative control|-|-| | + | |6: negative control|-|-|w|= |
| - | |7: positive contril|1100bp|-| | + | |7: positive contril|1100bp|-|w|= |
|}} | |}} | ||
| Line 513: | Line 512: | ||
|2: GFP2|854bp|-|f|= | |2: GFP2|854bp|-|f|= | ||
|3: GFP3|854bp|-|f|= | |3: GFP3|854bp|-|f|= | ||
| - | |4: riboswitch1+GFP| | + | |4: riboswitch1+GFP|1158bp|-|f|= |
| - | |5: riboswitch2+GFP| | + | |5: riboswitch2+GFP|1158bp|-|f|= |
| - | |6: riboswitch3+GFP| | + | |6: riboswitch3+GFP|1158bp|-|f|= |
|7: marker100 bp|-|-|n|= | |7: marker100 bp|-|-|n|= | ||
| - | |8: ribo1+vector| | + | |8: ribo1+vector|294bp|-|f|= |
| - | |9: ribo1+vector| | + | |9: ribo1+vector|294bp|-|f|= |
| - | |10: ribo2+vector| | + | |10: ribo2+vector|294bp|-|f|= |
| - | |11: ribo2+vector| | + | |11: ribo2+vector|294bp|-|f|= |
| - | |12: positive control|1100bp|-| | + | |12: positive control|1100bp|-|w|= |
| - | |13: negative control|-|-| | + | |13: negative control|-|-|w|= |
|}} | |}} | ||
| Line 556: | Line 555: | ||
|1: GFP1(J04630)|854bp|-|f|= | |1: GFP1(J04630)|854bp|-|f|= | ||
|2: GFP2(J04630)|854bp|-|f|= | |2: GFP2(J04630)|854bp|-|f|= | ||
| - | |3: Riboswitch1+vector| | + | |3: Riboswitch1+vector|294bp|-|f|= |
| - | |4: Riboswitch+2vector| | + | |4: Riboswitch+2vector|294bp|-|f|= |
| - | |5: | + | |5: marker 100 bp|-|-|n|= |
|6: GFP1(E0040)|720bp|-|f|= | |6: GFP1(E0040)|720bp|-|f|= | ||
| - | |7: GFP2(E0040)|720bp|-| | + | |7: GFP2(E0040)|720bp|-|f|= |
| - | |8: ribo1+vector| | + | |8: ribo1+vector|294bp|-|f|= |
| - | |9: ribo1+vector| | + | |9: ribo1+vector|294bp|-|f|= |
| - | |10: ribo2+vector| | + | |10: ribo2+vector|294bp|-|f|= |
| - | |11: ribo2+vector| | + | |11: ribo2+vector|294bp|-|f|= |
| - | |12: positive control|1100bp|-| | + | |12: positive control|1100bp|-|w|= |
| - | |13: negative control|-|-| | + | |13: negative control|-|-|w|= |
|}} | |}} | ||
| Line 588: | Line 587: | ||
![[Image:NYMU P9010281.jpg]] !! | ![[Image:NYMU P9010281.jpg]] !! | ||
{{:Team:NYMU-Taipei/GELC|= | {{:Team:NYMU-Taipei/GELC|= | ||
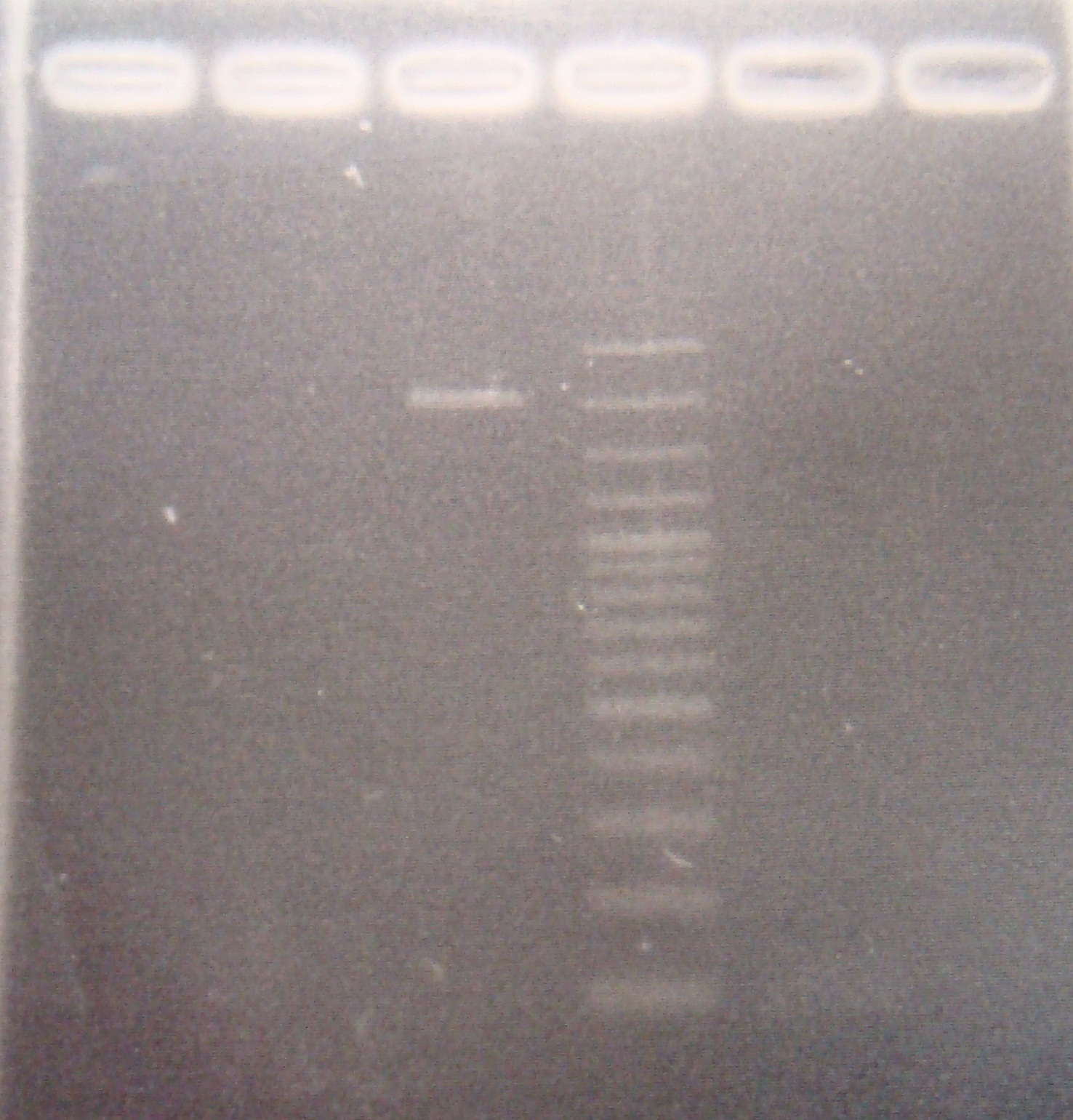
| - | |1: GFP+terminator(19f)|854bp|-| | + | |1: GFP+terminator(19f)|854bp|-|f|= |
| - | |2: GFP(E0040)|720bp|-| | + | |2: GFP(E0040)|720bp|-|f|= |
| - | |3: GFP(E0040)|720bp|-| | + | |3: GFP(E0040)|720bp|-|f|= |
|4: marker 100bp|-|-|n|= | |4: marker 100bp|-|-|n|= | ||
| - | |5: Positive Control|1100 bp|| | + | |5: Positive Control|1100 bp||w|= |
| - | |6: Negative Control||| | + | |6: Negative Control|||w|= |
|}} | |}} | ||
|} | |} | ||
| Line 608: | Line 607: | ||
|3: marker 100bp|-|-|n|= | |3: marker 100bp|-|-|n|= | ||
|4: -|-|-|n|= | |4: -|-|-|n|= | ||
| - | |5: (GFP)E0040-2|745bp|| | + | |5: (GFP)E0040-2|745bp||f|= |
| - | |6: (GFP)E0040-1|745bp|| | + | |6: (GFP)E0040-1|745bp||f|= |
| - | |7: (GFP)J04630|856bp|| | + | |7: (GFP)J04630|856bp||f|= |
| Line 640: | Line 639: | ||
![[Image:NYMU P9010283.jpg]] !! | ![[Image:NYMU P9010283.jpg]] !! | ||
{{:Team:NYMU-Taipei/GELC|= | {{:Team:NYMU-Taipei/GELC|= | ||
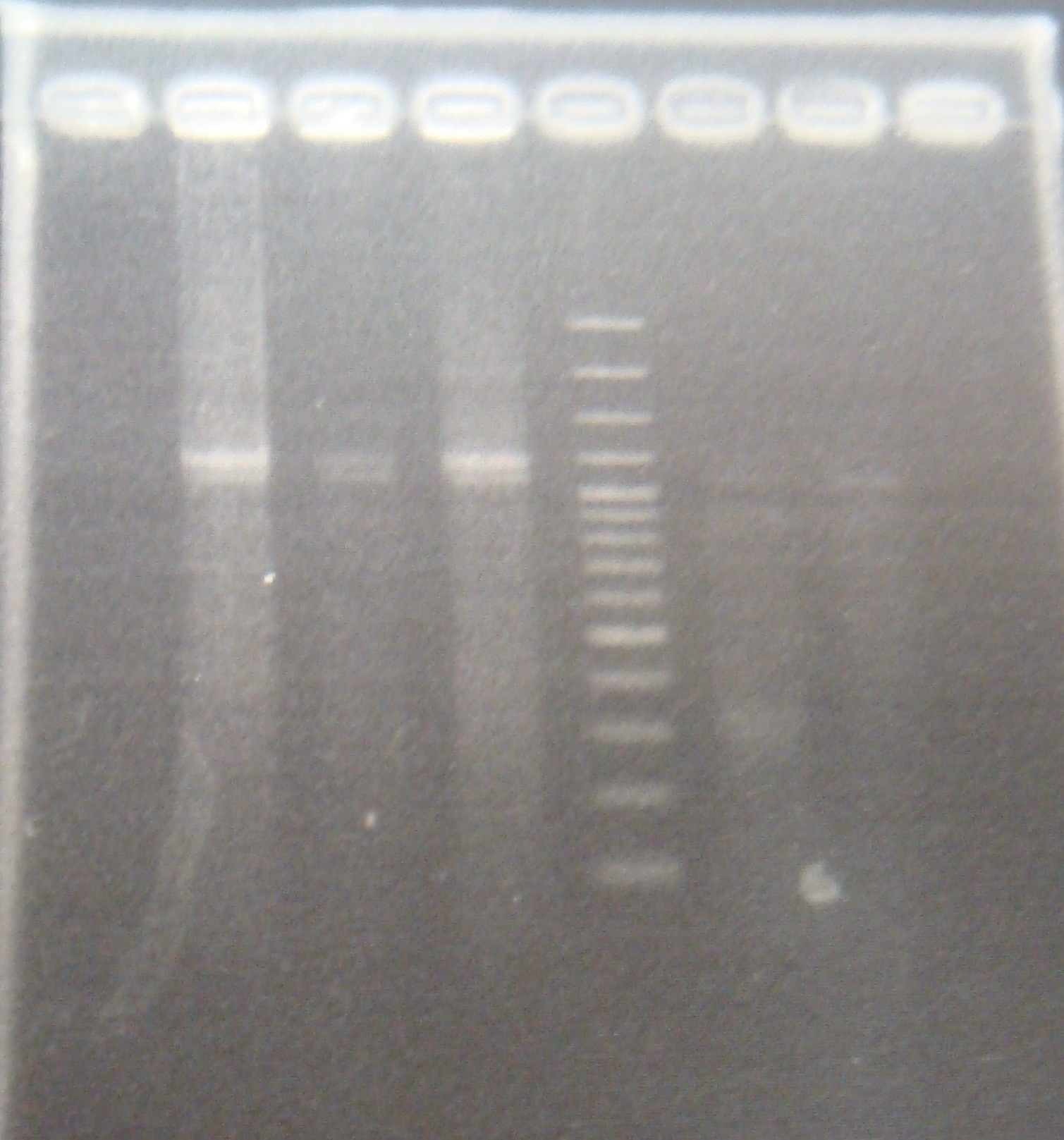
| - | |1: | + | |1: PFRV(1-a)|1158bp|294bp|f|= |
| - | |2: | + | |2: PFRV(1-b)|1158bp|294bp|f|= |
| - | |3: | + | |3: PFRV(2-a)|1158bp|294bp|f|= |
| - | |4: | + | |4: PFRV(2-b)|1158bp|294bp|f|= |
| - | |5: | + | |5: PFRV(3-a)|1158bp|294bp|f|= |
| - | |6: | + | |6: PFRV(3-b)|1158bp|294bp|f|= |
|7: -|-|-| | |7: -|-|-| | ||
|8: marker 100bp|-|-|n|= | |8: marker 100bp|-|-|n|= | ||
| - | |9: | + | |9: PFRV|1366bp|-|f|= |
| - | |10: | + | |10: PFRV|1366bp|-|f|= |
| - | |11: | + | |11: PFRV|1366bp|-|f| |
| - | |12: | + | |12: PFRV|1366bp|-|f|= |
| - | |13: | + | |13: PFRV|1366bp|-|f|= |
| - | |14: | + | |14: PFRV|1366bp|-|f|= |
| - | |15: positive control|1100bp| | + | |15: positive control|1100bp||w|= |
| - | |16: negative control|-|containment| | + | |16: negative control|-|containment|w|= |
|}} | |}} | ||
| Line 690: | Line 689: | ||
![[Image:NYMU P9070329.jpg]] !! | ![[Image:NYMU P9070329.jpg]] !! | ||
{{:Team:NYMU-Taipei/GELC|= | {{:Team:NYMU-Taipei/GELC|= | ||
| - | |1: FRV1| | + | |1: FRV1|1158bp|294bp|f|= |
| - | |2: FRV2| | + | |2: FRV2|1158bp|294bp|f|= |
| - | |3: marker | + | |3: marker 1kb|-|-|n|= |
| - | |4: FRV3| | + | |4: FRV3|1158bp|-|f|= |
| - | |5: FRV4| | + | |5: FRV4|1158bp|294bp|f|= |
| - | |6: positive control|1100bp|| | + | |6: positive control|1100bp||w|= |
| - | |7: negative control|| | + | |7: negative control||-|w|= |
| Line 727: | Line 726: | ||
![[Image:NYMU P9070327.jpg]] !! | ![[Image:NYMU P9070327.jpg]] !! | ||
{{:Team:NYMU-Taipei/GELC|= | {{:Team:NYMU-Taipei/GELC|= | ||
| - | |1: FRV1| | + | |1: FRV1|1158bp|294bp|f|= |
| - | |2: FRV2| | + | |2: FRV2|1158bp|294bp|f|= |
| - | |3: marker 100bp|-|-|n|= | + | |3: marker 100bp |-|-|n|= |
| - | |4: FRV3| | + | |4: FRV3|1158bp|294bp|f|= |
| - | |5: | + | |5: positive control|1000bp|-|w|= |
| - | |6 | + | |6: negative control|-|-|w|= |
| - | + | ||
| - | + | ||
|}} | |}} | ||
| Line 772: | Line 769: | ||
![[Image:NYMU_DSC05719.JPG|250px]] !! | ![[Image:NYMU_DSC05719.JPG|250px]] !! | ||
{{:Team:NYMU-Taipei/GELC|= | {{:Team:NYMU-Taipei/GELC|= | ||
| - | |1: | + | |1: positive control|1100bp|-|w|= |
| - | |2: | + | |2: negative control|-|-|w|= |
| - | |3: | + | |3: 100bp marker|-|-|f|= |
| - | |4: | + | |4: FRV|1158bp|1700bp|f|= |
| - | |5: | + | |5: FRV|1158bp|1700bp|f|= |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| + | | | ||
|}} | |}} | ||
{| border=1 | {| border=1 | ||
! Total:(*2) !! 50μl | ! Total:(*2) !! 50μl | ||
|- | |- | ||
| - | | template || | + | | template || 1μl |
|- | |- | ||
| - | | VF+VR || 2μl | + | | VF+VR || 2μl |
|- | |- | ||
| dNTP || 2μl | | dNTP || 2μl | ||
| Line 823: | Line 811: | ||
![[Image:NYMU_DSC05720.JPG|250px]] !! | ![[Image:NYMU_DSC05720.JPG|250px]] !! | ||
{{:Team:NYMU-Taipei/GELC|= | {{:Team:NYMU-Taipei/GELC|= | ||
| - | |1: | + | |1: FRV|1158bp|-|f|= |
| - | |2: | + | |2: FRV|1158bp|-|f|= |
| - | |3: | + | |3: FRV|1158bp|-|f|= |
| - | |4: | + | |4: FRV|1158bp|-|f|= |
| - | |5: | + | |5: 100bp marker|-|-|n|= |
| - | |6: | + | |6: FRV|1158bp|-|f|= |
| - | |7: | + | |7: FRV|1158bp|294bp|f|= |
| - | |8: | + | |8: positive control|1100bp|-|w|= |
| - | |9: | + | |9: negative control|-|-|w|= |
| - | + | | | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
|}} | |}} | ||
| Line 864: | Line 847: | ||
*Transformation 17:00 | *Transformation 17:00 | ||
=2010.09.09= | =2010.09.09= | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
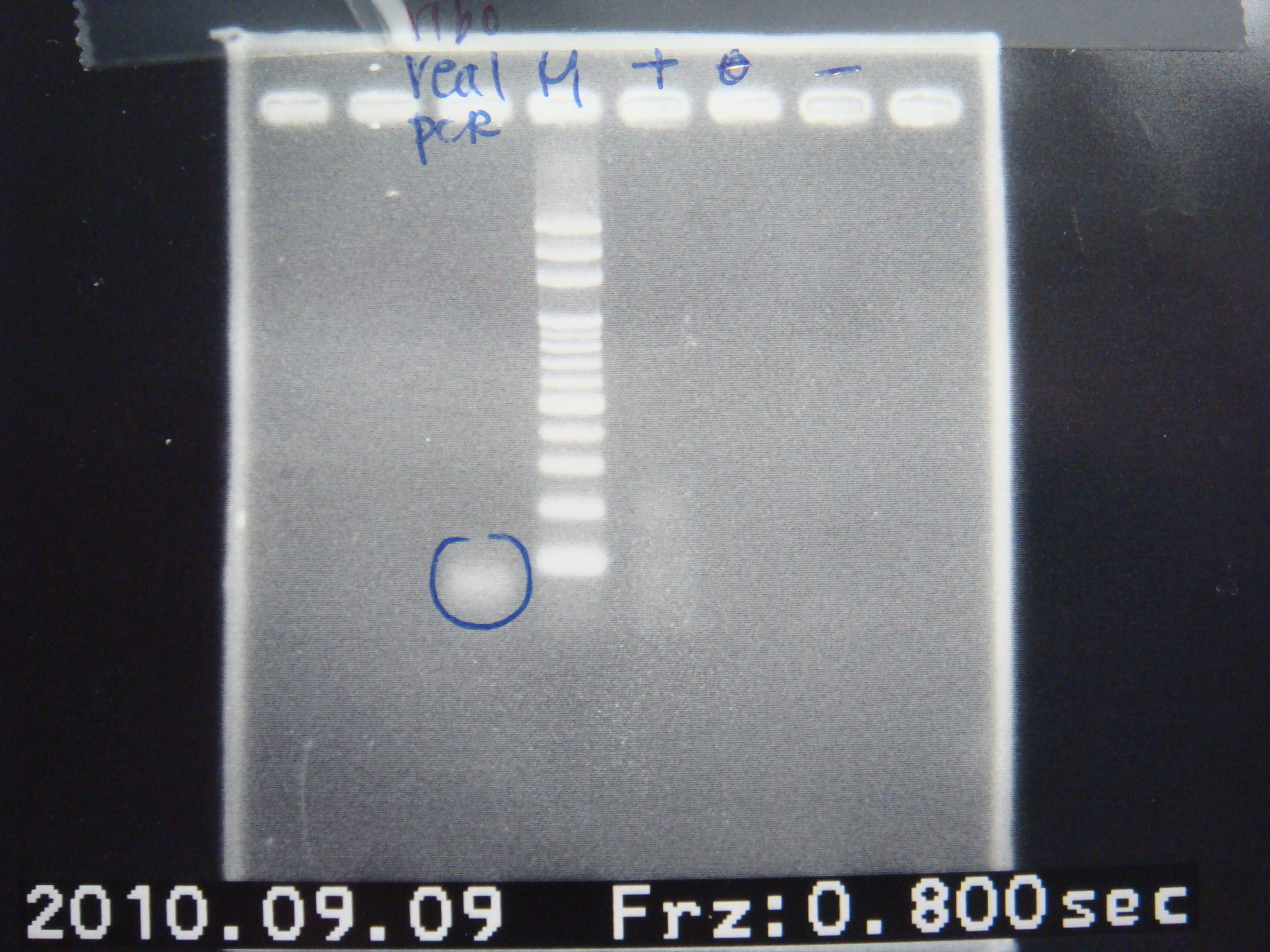
*Real PCR | *Real PCR | ||
| Line 881: | Line 859: | ||
| template || 2μl | | template || 2μl | ||
|- | |- | ||
| - | | | + | | FP+RP || 2μl |
|- | |- | ||
| dNTP || 2μl | | dNTP || 2μl | ||
| Line 889: | Line 867: | ||
| ddH2O || 39.75μl | | ddH2O || 39.75μl | ||
|- | |- | ||
| - | | | + | | pfu || 0.25μl |
|} | |} | ||
{| border="2" | {| border="2" | ||
| Line 899: | Line 877: | ||
| 94 || 15s | | 94 || 15s | ||
|- | |- | ||
| - | | | + | | 50.8 ||20s |
|- | |- | ||
| 74 || 45s | | 74 || 45s | ||
| Line 908: | Line 886: | ||
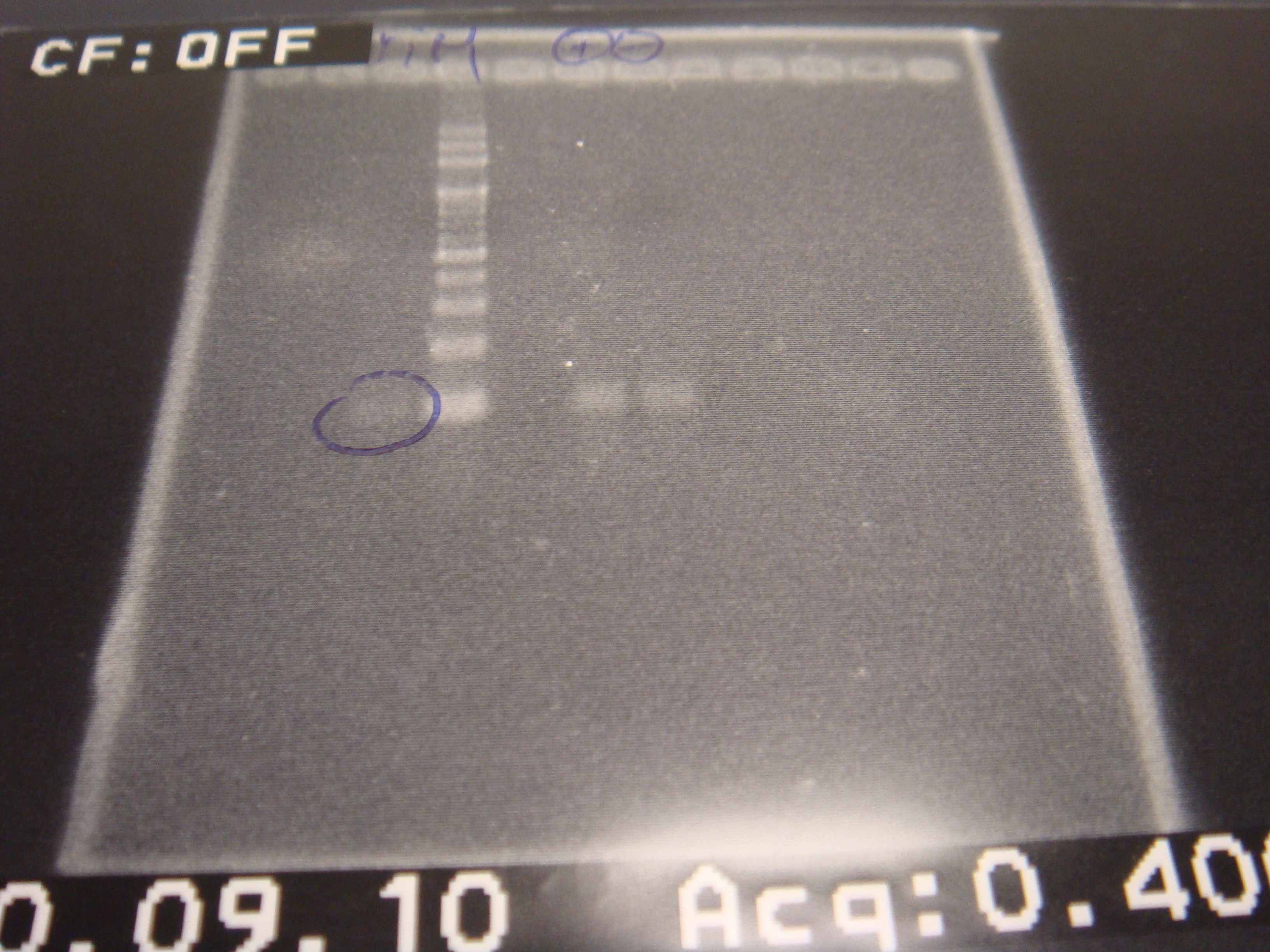
=2010.09.10= | =2010.09.10= | ||
| - | *real PCR | + | *real PCR (temperature test) |
*run gel | *run gel | ||
| Line 920: | Line 898: | ||
| template || 2μl | | template || 2μl | ||
|- | |- | ||
| - | | | + | | FP+RP || 2μl |
|- | |- | ||
| dNTP || 2μl | | dNTP || 2μl | ||
| Line 928: | Line 906: | ||
| ddH2O || 39.75μl | | ddH2O || 39.75μl | ||
|- | |- | ||
| - | | | + | | pfu || 0.25μl |
|} | |} | ||
{| border="2" | {| border="2" | ||
| Line 938: | Line 916: | ||
| 94 || 15s | | 94 || 15s | ||
|- | |- | ||
| - | | | + | | 50.8 ||20s |
|- | |- | ||
| 74 || 45s | | 74 || 45s | ||
| Line 953: | Line 931: | ||
*transform | *transform | ||
------------------------ | ------------------------ | ||
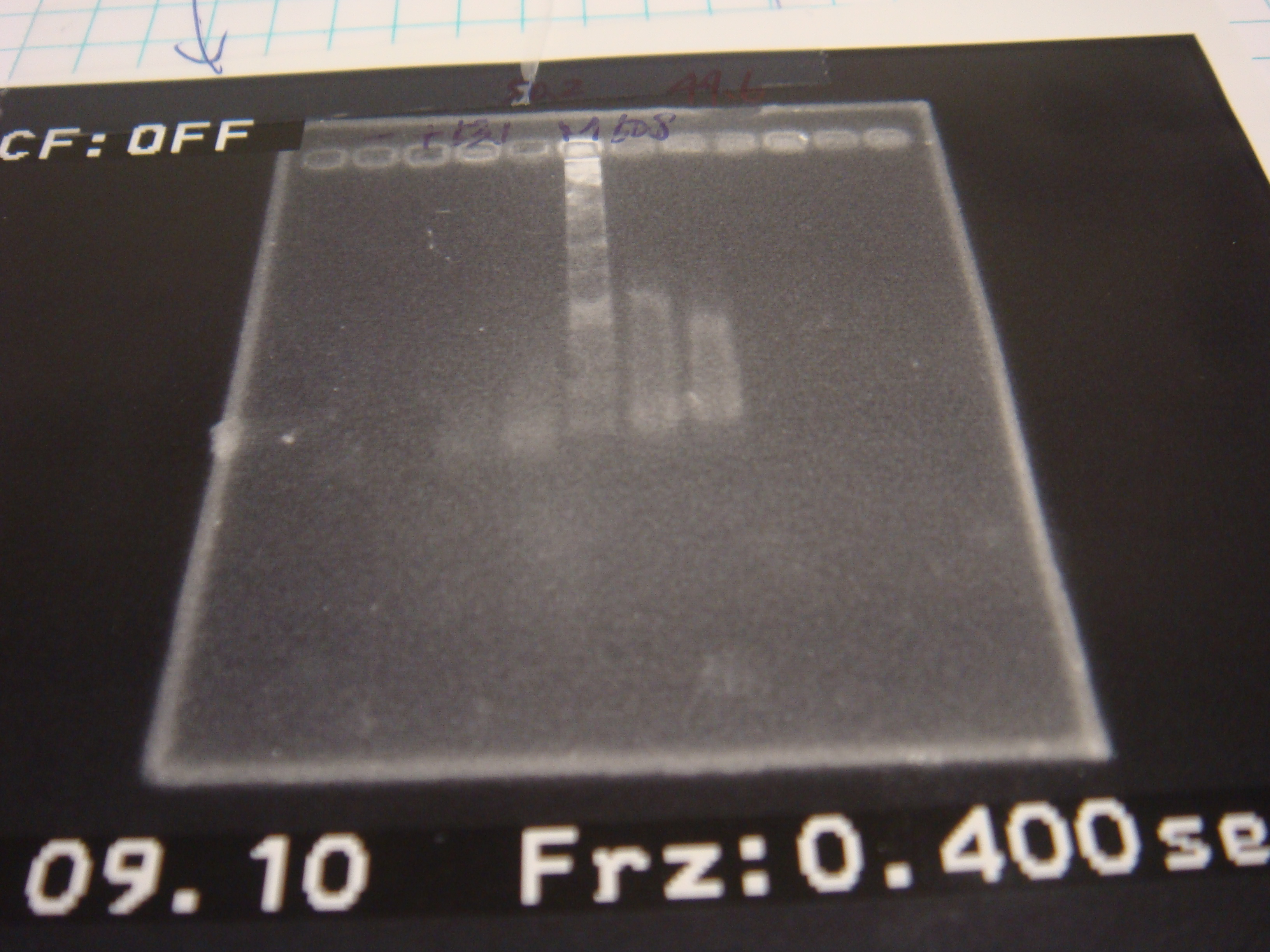
| - | *real pcr for 2 tubes | + | *real pcr for 2 tubes(in suitable temperature) |
| + | {| border=1|- | ||
| + | ![[Image:NYMU_DSC05723.JPG|250px]] !! | ||
| + | |||
| + | |||
| + | {| border="1" | ||
| + | ! Total:(*2) !! 50μl | ||
| + | |- | ||
| + | | template || 2μl | ||
| + | |- | ||
| + | | FP+RP || 2μl | ||
| + | |- | ||
| + | | dNTP || 2μl | ||
| + | |- | ||
| + | | Buffer || 5μl | ||
| + | |- | ||
| + | | ddH2O || 39.75μl | ||
| + | |- | ||
| + | | pfu || 0.25μl | ||
| + | |} | ||
| + | {| border="2" | ||
| + | |- | ||
| + | | Temp || Time | ||
| + | |- | ||
| + | | 94 || 60s | ||
| + | |- | ||
| + | | 94 || 15s | ||
| + | |- | ||
| + | | 50.8 ||20s | ||
| + | |- | ||
| + | | 74 || 45s | ||
| + | |- | ||
| + | | 74 || 300s | ||
| + | |} | ||
| + | |} | ||
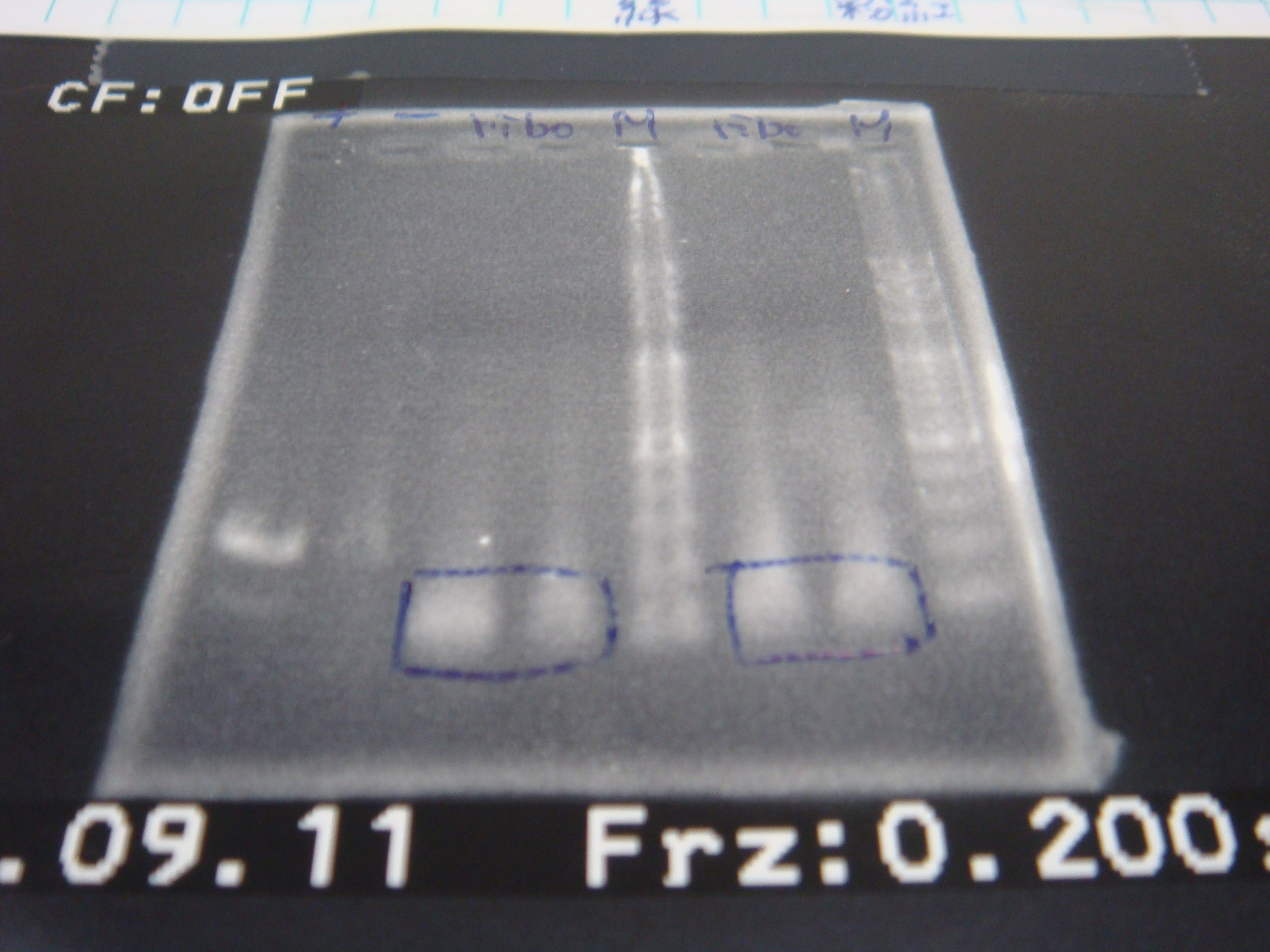
=2010.09.11= | =2010.09.11= | ||
| Line 959: | Line 971: | ||
------------------------ | ------------------------ | ||
*two real tubes run gel and cut gel and gel extraction | *two real tubes run gel and cut gel and gel extraction | ||
| + | [[Image:NYMU_DSC05725.JPG|250px]] | ||
| + | |||
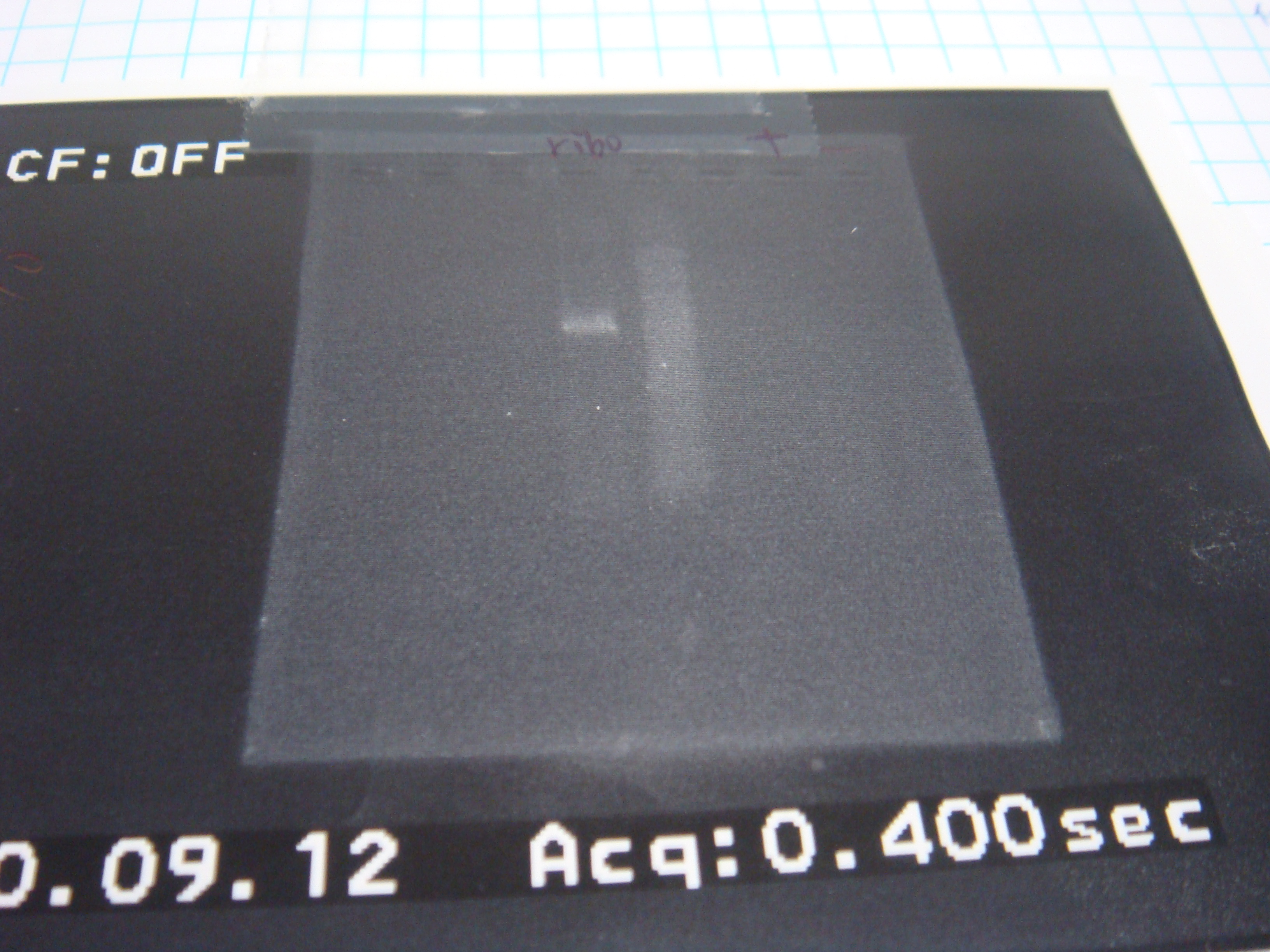
=2010.09.12= | =2010.09.12= | ||
*run gel(length isn't correct) | *run gel(length isn't correct) | ||
| + | [[Image:NYMU_DSC05726.JPG|250px]] | ||
*(real PCR) digestion 12:00 | *(real PCR) digestion 12:00 | ||
*ligation 15:00 | *ligation 15:00 | ||
*transform 19:40 | *transform 19:40 | ||
| + | |||
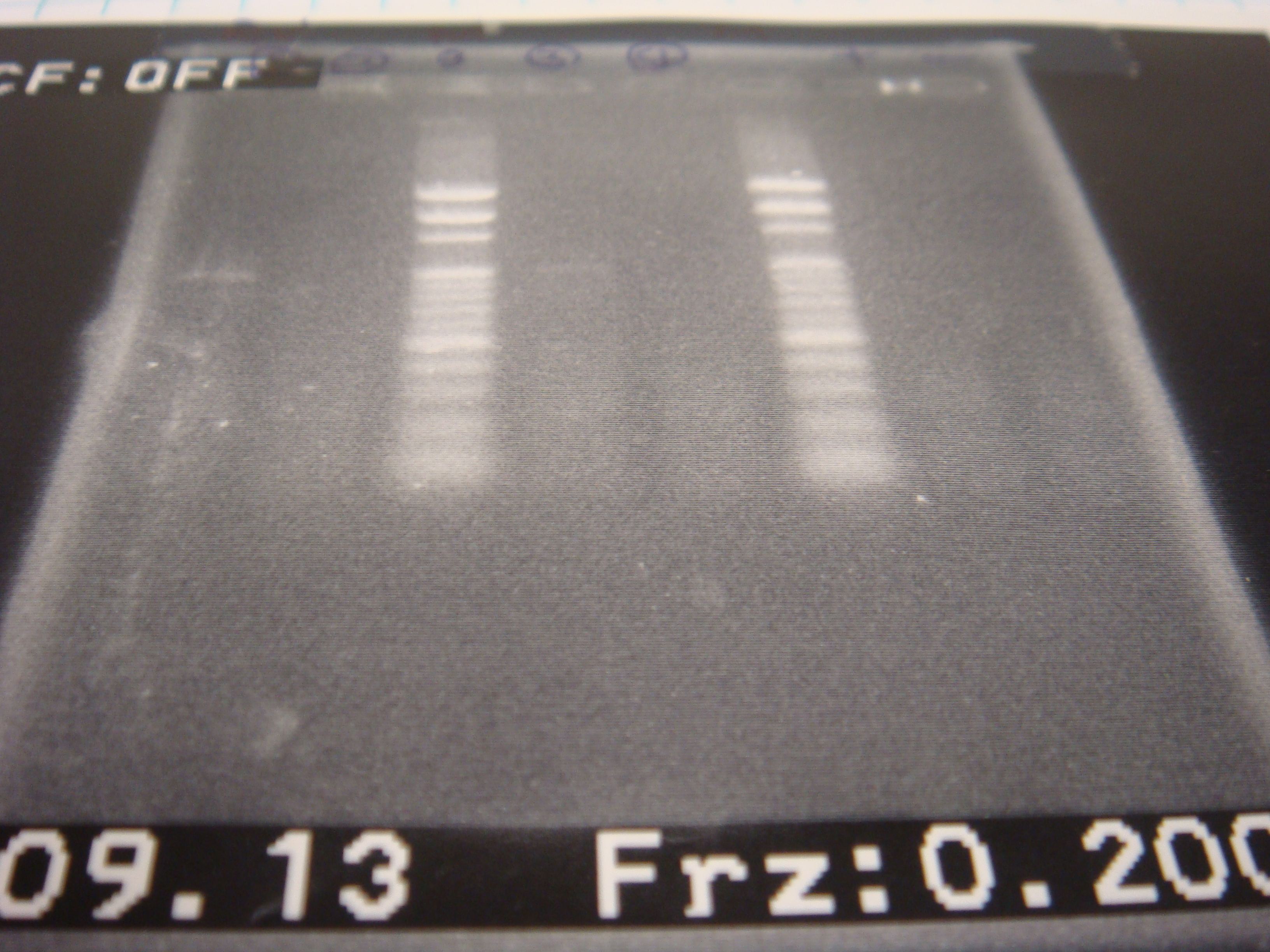
=2010.09.13= | =2010.09.13= | ||
*colony PCR 13:25 (3 in 1) | *colony PCR 13:25 (3 in 1) | ||
| + | {| border=1|- | ||
| + | ![[Image:NYMU_DSC05728.JPG|250px]] !! | ||
| + | |||
| + | |||
| + | {| border="1" | ||
| + | ! Total:(*2) !! 50μl | ||
| + | |- | ||
| + | | template || 2μl | ||
| + | |- | ||
| + | | VR+VF || 2μl | ||
| + | |- | ||
| + | | dNTP || 2μl | ||
| + | |- | ||
| + | | Buffer || 5μl | ||
| + | |- | ||
| + | | ddH2O || 39.75μl | ||
| + | |- | ||
| + | | tag || 0.25μl | ||
| + | |} | ||
| + | {| border="2" | ||
| + | |- | ||
| + | | Temp || Time | ||
| + | |- | ||
| + | | 94 || 60s | ||
| + | |- | ||
| + | | 94 || 15s | ||
| + | |- | ||
| + | | 55 ||20s | ||
| + | |- | ||
| + | | 72 || 30s ||30 cycle | ||
| + | |- | ||
| + | | 72 || 300s | ||
| + | |} | ||
| + | |} | ||
---------------------- | ---------------------- | ||
*nanodrop | *nanodrop | ||
*ligation 14:00 | *ligation 14:00 | ||
*Colony PCR 15:00 | *Colony PCR 15:00 | ||
| - | |||
=2010.09.22 = | =2010.09.22 = | ||
| Line 978: | Line 1,027: | ||
{| border="1" | {| border="1" | ||
|- | |- | ||
| - | ! Total: !! 49μl || X1.7 | + | ! Total: !! 49μl || X1.7 || |
|- | |- | ||
| FP || 1μl || 1.7μl || Theophylline Riboswitch Forward Primer | | FP || 1μl || 1.7μl || Theophylline Riboswitch Forward Primer | ||
| Line 1,032: | Line 1,081: | ||
{| border="1" | {| border="1" | ||
|- | |- | ||
| - | ! Total: !! 49μl || X1.9 | + | ! Total: !! 49μl || X1.9 |
|- | |- | ||
| VR+VF|| 2μl || 3.8μl | | VR+VF|| 2μl || 3.8μl | ||
| Line 1,081: | Line 1,130: | ||
{| border="1" | {| border="1" | ||
|- | |- | ||
| - | ! Total: !! 49μl || X2.5 | + | ! Total: !! 49μl || X2.5 |
|- | |- | ||
| VR+VF|| 2μl || 5μl | | VR+VF|| 2μl || 5μl | ||
| Line 1,089: | Line 1,138: | ||
| 10XBuff. || 5μl || 12.5μl | | 10XBuff. || 5μl || 12.5μl | ||
|- | |- | ||
| - | | tag || 0.25μl || 1. | + | | tag || 0.25μl || 1.25μl |
|- | |- | ||
| ddH2O || 39.75μl || 99.375μl | | ddH2O || 39.75μl || 99.375μl | ||
| Line 1,148: | Line 1,197: | ||
{| border="1" | {| border="1" | ||
|- | |- | ||
| - | ! Total: !! 49μl || X1.6 | + | ! Total: !! 49μl || X1.6 |
|- | |- | ||
| VR+VF|| 2μl || 3.2μl | | VR+VF|| 2μl || 3.2μl | ||
| Line 1,156: | Line 1,205: | ||
| 10XBuff. || 5μl || 8μl | | 10XBuff. || 5μl || 8μl | ||
|- | |- | ||
| - | | tag || 0.25μl || 0. | + | | tag || 0.25μl || 0.4μl |
|- | |- | ||
| ddH2O || 39.75μl || 63.6μl | | ddH2O || 39.75μl || 63.6μl | ||
| Line 1,198: | Line 1,247: | ||
{| border="1" | {| border="1" | ||
|- | |- | ||
| - | ! Total: !! 49μl || X1 | + | ! Total: !! 49μl || X1.9 |
|- | |- | ||
| VR+VF|| 2μl || 3.8μl | | VR+VF|| 2μl || 3.8μl | ||
| Line 1,206: | Line 1,255: | ||
| 10XBuff. || 5μl || 9.5μl | | 10XBuff. || 5μl || 9.5μl | ||
|- | |- | ||
| - | | tag || 0.25μl || 0. | + | | tag || 0.25μl || 0.475μl |
|- | |- | ||
| ddH2O || 39.75μl || 75.525μl | | ddH2O || 39.75μl || 75.525μl | ||
| Line 1,252: | Line 1,301: | ||
{| border="1" | {| border="1" | ||
|- | |- | ||
| - | + | | Total: || 49μl || X2.5 | |
|- | |- | ||
| VR+VF|| 2μl || 5μl | | VR+VF|| 2μl || 5μl | ||
| Line 1,260: | Line 1,309: | ||
| 10XBuff. || 5μl || 12.5μl | | 10XBuff. || 5μl || 12.5μl | ||
|- | |- | ||
| - | | tag || 0.25μl || 0. | + | | tag || 0.25μl || 0.625μl |
|- | |- | ||
| ddH2O || 39.75μl || 99.375μl | | ddH2O || 39.75μl || 99.375μl | ||
| Line 1,312: | Line 1,361: | ||
{| border="1" | {| border="1" | ||
|- | |- | ||
| - | ! Total: !! 49μl || | + | ! Total: !! 49μl || X3.8 |
|- | |- | ||
| VR+VF|| 2μl || μl | | VR+VF|| 2μl || μl | ||
| Line 1,359: | Line 1,408: | ||
{| border="1" | {| border="1" | ||
|- | |- | ||
| - | ! Total: !! 49μl || X3.8 | + | ! Total: !! 49μl || X3.8 |
|- | |- | ||
| VR+VF|| 2μl || 7.6μl | | VR+VF|| 2μl || 7.6μl | ||
| Line 1,367: | Line 1,416: | ||
| 10XBuff. || 5μl || 17μl | | 10XBuff. || 5μl || 17μl | ||
|- | |- | ||
| - | | tag || 0.25μl || 0. | + | | tag || 0.25μl || 0.95μl |
|- | |- | ||
| ddH2O || 39.75μl || 151.06μl | | ddH2O || 39.75μl || 151.06μl | ||
| Line 1,401: | Line 1,450: | ||
{| border="1" | {| border="1" | ||
|- | |- | ||
| - | ! Total: !! 49μl || X3.8 | + | ! Total: !! 49μl || X3.8 |
|- | |- | ||
| VR+VF|| 2μl || 7.6μl | | VR+VF|| 2μl || 7.6μl | ||
| Line 1,409: | Line 1,458: | ||
| 10XBuff. || 5μl || 17μl | | 10XBuff. || 5μl || 17μl | ||
|- | |- | ||
| - | | tag || 0.25μl || 0. | + | | tag || 0.25μl || 0.95μl |
|- | |- | ||
| ddH2O || 39.75μl || 151.06μl | | ddH2O || 39.75μl || 151.06μl | ||
| Line 1,447: | Line 1,496: | ||
{| border="1" | {| border="1" | ||
|- | |- | ||
| - | ! Total: !! 49μl || X3.2 | + | ! Total: !! 49μl || X3.2 |
|- | |- | ||
| VR+VF|| 2μl || 6.4μl | | VR+VF|| 2μl || 6.4μl | ||
| Line 1,455: | Line 1,504: | ||
| 10XBuff. || 5μl || 15μl | | 10XBuff. || 5μl || 15μl | ||
|- | |- | ||
| - | | tag || 0.25μl || 0. | + | | tag || 0.25μl || 0.8μl |
|- | |- | ||
| ddH2O || 39.75μl || 127.2μl | | ddH2O || 39.75μl || 127.2μl | ||
| Line 1,506: | Line 1,555: | ||
{| border="1" | {| border="1" | ||
|- | |- | ||
| - | ! Total: !! 49μl || X1.7 | + | ! Total: !! 49μl || X1.7 |
|- | |- | ||
| VR+VF|| 2μl || 3.4μl | | VR+VF|| 2μl || 3.4μl | ||
| Line 1,514: | Line 1,563: | ||
| 10XBuff. || 5μl || 8μl | | 10XBuff. || 5μl || 8μl | ||
|- | |- | ||
| - | | tag || 0.25μl || 0. | + | | tag || 0.25μl || 0.425μl |
|- | |- | ||
| ddH2O || 39.75μl || 67.575μl | | ddH2O || 39.75μl || 67.575μl | ||
| Line 1,563: | Line 1,612: | ||
{| border="1" | {| border="1" | ||
|- | |- | ||
| - | ! Total: !! 49μl || X2.5 | + | ! Total: !! 49μl || X2.5 |
|- | |- | ||
| VR+VF|| 2μl || 5μl | | VR+VF|| 2μl || 5μl | ||
| Line 1,571: | Line 1,620: | ||
| 10XBuff. || 5μl || 12.5μl | | 10XBuff. || 5μl || 12.5μl | ||
|- | |- | ||
| - | | tag || 0.25μl || 0. | + | | tag || 0.25μl || 0.625μl |
|- | |- | ||
| ddH2O || 39.75μl || 99.375μl | | ddH2O || 39.75μl || 99.375μl | ||
| Line 1,607: | Line 1,656: | ||
{| border="1" | {| border="1" | ||
|- | |- | ||
| - | ! Total: !! 49μl || X2.5 | + | ! Total: !! 49μl || X2.5 |
|- | |- | ||
| VR+VF|| 2μl || 5μl | | VR+VF|| 2μl || 5μl | ||
| Line 1,615: | Line 1,664: | ||
| 10XBuff. || 5μl || 12.5μl | | 10XBuff. || 5μl || 12.5μl | ||
|- | |- | ||
| - | | tag || 0.25μl || 0. | + | | tag || 0.25μl || 0.625μl |
|- | |- | ||
| ddH2O || 39.75μl || 99.375μl | | ddH2O || 39.75μl || 99.375μl | ||
| Line 1,672: | Line 1,721: | ||
{| border="1" | {| border="1" | ||
|- | |- | ||
| - | ! Total: !! 49μl || X1.3 | + | ! Total: !! 49μl || X1.3 |
|- | |- | ||
| VR+VF|| 2μl || 2.6μl | | VR+VF|| 2μl || 2.6μl | ||
| Line 1,680: | Line 1,729: | ||
| 10XBuff. || 5μl || 6.5μl | | 10XBuff. || 5μl || 6.5μl | ||
|- | |- | ||
| - | | tag || 0.25μl || 0. | + | | tag || 0.25μl || 0.325μl |
|- | |- | ||
| ddH2O || 39.75μl || 51.675μl | | ddH2O || 39.75μl || 51.675μl | ||
| Line 1,731: | Line 1,780: | ||
|} | |} | ||
| - | **Because Theophylline entry into E. coli spend | + | **Because Theophylline entry into E. coli spend 1 hours at least, O.D= 0.0325 |
*Assay:add 80 μl PFRC liquid into LB | *Assay:add 80 μl PFRC liquid into LB | ||
{| border=1 | {| border=1 | ||
| Line 1,756: | Line 1,805: | ||
{| border="1" | {| border="1" | ||
|- | |- | ||
| - | ! Total: !! 49μl || X2.1 | + | ! Total: !! 49μl || X2.1 |
|- | |- | ||
| VR+VF|| 2μl || 4.2μl | | VR+VF|| 2μl || 4.2μl | ||
| Line 1,764: | Line 1,813: | ||
| 10XBuff. || 5μl || 10.5μl | | 10XBuff. || 5μl || 10.5μl | ||
|- | |- | ||
| - | | tag || 0.25μl || 0. | + | | tag || 0.25μl || 0.525μl |
|- | |- | ||
| ddH2O || 39.75μl || 83.475μl | | ddH2O || 39.75μl || 83.475μl | ||
| Line 1,860: | Line 1,909: | ||
{| border="1" | {| border="1" | ||
|- | |- | ||
| - | ! Total: !! 49μl || X2.5 | + | ! Total: !! 49μl || X2.5 |
|- | |- | ||
| VR+VF|| 2μl || 5μl | | VR+VF|| 2μl || 5μl | ||
| Line 1,868: | Line 1,917: | ||
| 10XBuff. || 5μl || 12.5μl | | 10XBuff. || 5μl || 12.5μl | ||
|- | |- | ||
| - | | tag || 0.25μl || 1. | + | | tag || 0.25μl || 1.25μl |
|- | |- | ||
| ddH2O || 39.75μl || 99.375μl | | ddH2O || 39.75μl || 99.375μl | ||
| Line 1,904: | Line 1,953: | ||
*Transform | *Transform | ||
------------------------------- | ------------------------------- | ||
| - | *Theophylline solution | + | *Preparing 0.1 M Theophylline solution in DMSO. |
*Assay | *Assay | ||
{| border=1 | {| border=1 | ||
| Line 1,934: | Line 1,983: | ||
| K || 20000 μM || 800 μl || 117.7 ||4 | | K || 20000 μM || 800 μl || 117.7 ||4 | ||
|} | |} | ||
| + | |||
| + | * Modified experiment design | ||
| + | |||
{| border=1 | {| border=1 | ||
|- | |- | ||
| - | ! !! Theophylline concentration || Theophylline(0.1M) || PFRC liquid(μl)|| Cm50(μl) | + | ! !! Theophylline concentration || Theophylline(0.1M) || PFRC liquid(μl)||FRC liquid(μl)|| Cm50(μl) |
|- | |- | ||
| - | | O || 0 μM || 0|| 261 || 4 | + | | O || 0 μM || 0 || 261 ||0|| 4 |
|- | |- | ||
| - | | A || 10 μM || 0.4 μl || 261 ||4 | + | | A || 10 μM || 0.4 μl || 261 ||0|| 4 |
|- | |- | ||
| - | | B || 50 μM || 2 μl || 261 ||4 | + | | B || 50 μM || 2 μl || 261 ||0|| 4 |
|- | |- | ||
| - | | C || 100 μM || 4 μl || 261 ||4 | + | | C || 100 μM || 4 μl ||261 ||0|| 4 |
|- | |- | ||
| - | | D || 200 μM || 8 μl || 261 ||4 | + | | D || 200 μM || 8 μl || 261 ||0||4 |
|- | |- | ||
| - | | E || 500 μM || 20 μl || 261 ||4 | + | | E || 500 μM || 20 μl || 261 ||0||4 |
|- | |- | ||
| - | | F || 1000 μM || 40 μpl || 261 ||4 | + | | F || 1000 μM || 40 μpl || 261 ||0||4 |
|- | |- | ||
| - | | G || 2000 μM || 80 μl || 261 ||4 | + | | G || 2000 μM || 80 μl || 261 ||0||4 |
|- | |- | ||
| - | | H || 4000 μM || 160 μl || 261 ||4 | + | | H || 4000 μM || 160 μl || 261 ||0||4 |
|- | |- | ||
| - | | I || | + | | I || 8000 μM || 320 μl || 261 ||0||4 |
|- | |- | ||
| - | | J || 10000 μM || 400 μl || 261 ||4 | + | | J || 10000 μM || 400 μl ||261 ||0||4 |
|- | |- | ||
| - | | K || 20000 μM || 800 μl || 261 ||4 | + | | K || 20000 μM || 800 μl || 261 ||0||4 |
| - | |- | + | |- |
| - | | NT || 100 μM | + | | NT || 100 μM || 4 μl || 0 || 261 || 4 |
| - | |- | + | |- |
| - | | N || 0 μM || 0 μl || 261 ||4 | + | | N || 0 μM || 0 μl || 0 || 261 || 4 |
| + | |- | ||
|} | |} | ||
| - | |||
| - | |||
| - | |||
=2010.10.24= | =2010.10.24= | ||
| Line 1,976: | Line 2,026: | ||
{| border="1" | {| border="1" | ||
|- | |- | ||
| - | ! Total: !! 49μl || X0.9 | + | ! Total: !! 49μl || X0.9 |
|- | |- | ||
| VR+VF|| 2μl || 1.8μl | | VR+VF|| 2μl || 1.8μl | ||
| Line 1,984: | Line 2,034: | ||
| 10XBuff. || 5μl || 4.5μl | | 10XBuff. || 5μl || 4.5μl | ||
|- | |- | ||
| - | | tag || 0.25μl || 0. | + | | tag || 0.25μl || 0.225μl |
|- | |- | ||
| ddH2O || 39.75μl || 35.775μl | | ddH2O || 39.75μl || 35.775μl | ||
| Line 2,019: | Line 2,069: | ||
{| border="1" | {| border="1" | ||
|- | |- | ||
| - | ! Total: !! 49μl || X1.6 | + | ! Total: !! 49μl || X1.6 |
|- | |- | ||
| VR+VF|| 2μl || 3.2μl | | VR+VF|| 2μl || 3.2μl | ||
| Line 2,027: | Line 2,077: | ||
| 10XBuff. || 5μl || 8μl | | 10XBuff. || 5μl || 8μl | ||
|- | |- | ||
| - | | tag || 0.25μl || 0. | + | | tag || 0.25μl || 0.8μl |
|- | |- | ||
| ddH2O || 39.75μl || 63μl | | ddH2O || 39.75μl || 63μl | ||
| Line 2,060: | Line 2,110: | ||
{| border=1 | {| border=1 | ||
|- | |- | ||
| - | ! !! Theophylline concentration || Theophylline(0.1M) || LB(μl) || PFRC liquid(μl)|| Cm50(μl) | + | ! !! Theophylline concentration || Theophylline(0.1M) || Remove LB(μl) || PFRC liquid(μl)||FRC liquid(μl)|| Cm50(μl) |
|- | |- | ||
| - | | O || 0 μM || 0 || 325 || 325 || 4 | + | | O || 0 μM || 0 || 325 || 325 ||0|| 4 |
|- | |- | ||
| - | | A || 10 μM || 0.4 μl || 325|| 325 ||4 | + | | A || 10 μM || 0.4 μl || 325|| 325 ||0|| 4 |
|- | |- | ||
| - | | B || 50 μM || 2 μl || 327||325 ||4 | + | | B || 50 μM || 2 μl || 327||325 ||0|| 4 |
|- | |- | ||
| - | | C || 100 μM || 4 μl ||329 ||325 ||4 | + | | C || 100 μM || 4 μl ||329 ||325 ||0|| 4 |
|- | |- | ||
| - | | D || 200 μM || 8 μl || 333||325 ||4 | + | | D || 200 μM || 8 μl || 333||325 ||0||4 |
|- | |- | ||
| - | | E || 500 μM || 20 μl || 345||325 ||4 | + | | E || 500 μM || 20 μl || 345||325 ||0||4 |
|- | |- | ||
| - | | F || 1000 μM || 40 μpl || 365||325 ||4 | + | | F || 1000 μM || 40 μpl || 365||325 ||0||4 |
|- | |- | ||
| - | | G || 2000 μM || 80 μl || 405||325 ||4 | + | | G || 2000 μM || 80 μl || 405||325 ||0||4 |
|- | |- | ||
| - | | H || 4000 μM || 160 μl || 485||325 ||4 | + | | H || 4000 μM || 160 μl || 485||325 ||0||4 |
|- | |- | ||
| - | | I || 8000 μM || 320 μl || 645||325 ||4 | + | | I || 8000 μM || 320 μl || 645||325 ||0||4 |
|- | |- | ||
| - | | J || 10000 μM || 400 μl ||725 ||325 ||4 | + | | J || 10000 μM || 400 μl ||725 ||325 ||0||4 |
|- | |- | ||
| - | | K || 20000 μM || 800 μl ||1125 ||325 ||4 | + | | K || 20000 μM || 800 μl ||1125 ||325 ||0||4 |
|- | |- | ||
| - | | NT || 100 μM || 4 μl || 504 || 500 | + | | NT || 100 μM || 4 μl || 504 || 0 || 500 || 4 |
|- | |- | ||
| - | | N || 0 μM || 0 μl ||500 ||500 | + | | N || 0 μM || 0 μl ||500 || 0 || 500 || 4 |
|- | |- | ||
|} | |} | ||
| Line 2,104: | Line 2,154: | ||
{| border="1" | {| border="1" | ||
|- | |- | ||
| - | ! Total: !! 49μl || X2.4 | + | ! Total: !! 49μl || X2.4 |
|- | |- | ||
| VR+VF|| 2μl || 4.8μl | | VR+VF|| 2μl || 4.8μl | ||
| Line 2,112: | Line 2,162: | ||
| 10XBuff. || 5μl || 12μl | | 10XBuff. || 5μl || 12μl | ||
|- | |- | ||
| - | | tag || 0.25μl || 0. | + | | tag || 0.25μl || 0.6μl |
|- | |- | ||
| ddH2O || 39.75μl || 95.4μl | | ddH2O || 39.75μl || 95.4μl | ||
| Line 2,147: | Line 2,197: | ||
{| border=1 | {| border=1 | ||
|- | |- | ||
| - | ! !! Theophylline concentration || Theophylline(0.1M) || LB(μl) || PFRC liquid(μl)|| Cm50(μl) | + | ! !! Theophylline concentration || Theophylline(0.1M) || Remove LB(μl) || PFRC liquid(μl)|| FRC liquid(μl)|| Cm50(μl) |
|- | |- | ||
| - | | O || 0 μM || 0 || 151 || 147 || 4 | + | | O || 0 μM || 0 || 151 || 147 || 0 || 4 |
|- | |- | ||
| - | | A || 50 μM || 2 μl || 153|| 147 ||4 | + | | A || 50 μM || 2 μl || 153|| 147 ||0 ||4 |
|- | |- | ||
| - | | B || 500 μM || 20 μl || 171||147 ||4 | + | | B || 500 μM || 20 μl || 171||147 ||0 ||4 |
|- | |- | ||
| - | | C || 1000 μM || 40 μl ||191 ||147 ||4 | + | | C || 1000 μM || 40 μl ||191 ||147 ||0 ||4 |
|- | |- | ||
| - | | D || 4000 μM || 160 μl || 311||147 ||4 | + | | D || 4000 μM || 160 μl || 311||147 ||0 ||4 |
|- | |- | ||
| - | | E || 10000 μM || 400 μl || 551||147 ||4 | + | | E || 10000 μM || 400 μl || 551||147 ||0 ||4 |
|- | |- | ||
| - | | NT || 100 μM || 4 μl ||158 || 150 | + | | NT || 100 μM || 4 μl ||158 || 0||150 || 4 |
|- | |- | ||
| - | | N || 0 μM || 0 μl ||154 ||150 | + | | N || 0 μM || 0 μl ||154 ||0||150 || 4 |
|- | |- | ||
|} | |} | ||
| Line 2,171: | Line 2,221: | ||
{| border=1 | {| border=1 | ||
|- | |- | ||
| - | ! !! Theophylline concentration || Theophylline(0.1M) || LB(μl) || PFRC liquid(μl)|| Cm50(μl) | + | ! !! Theophylline concentration || Theophylline(0.1M) || Remove LB(μl) || PFRC liquid(μl)|| FRC liquid(μl)|| Cm50(μl) |
|- | |- | ||
| - | | O || 0 μM || 0 || 151 || 147 || 4 | + | | O || 0 μM || 0 || 151 || 147 || 0 || 4 |
|- | |- | ||
| - | | A || 50 μM || 2 μl || 153|| 147 ||4 | + | | A || 50 μM || 2 μl || 153|| 147 ||0 ||4 |
|- | |- | ||
| - | | B || 500 μM || 20 μl || 171||147 ||4 | + | | B || 500 μM || 20 μl || 171||147 ||0 ||4 |
|- | |- | ||
| - | | C || 1000 μM || 40 μl ||191 ||147 ||4 | + | | C || 1000 μM || 40 μl ||191 ||147 ||0 ||4 |
|- | |- | ||
| - | | D || 4000 μM || 160 μl || 311||147 ||4 | + | | D || 4000 μM || 160 μl || 311||147 ||0 ||4 |
|- | |- | ||
| - | | E || 10000 μM || 400 μl || 551||147 ||4 | + | | E || 10000 μM || 400 μl || 551||147 ||0 ||4 |
|- | |- | ||
| - | | NT || 100 μM || 4 μl ||158 || 150 | + | | NT || 100 μM || 4 μl ||158 || 0||150 || 4 |
|- | |- | ||
| - | | N || 0 μM || 0 μl ||154 ||150 | + | | N || 0 μM || 0 μl ||154 ||0||150 || 4 |
|- | |- | ||
|} | |} | ||
{{:Team:NYMU-Taipei/Footer}} | {{:Team:NYMU-Taipei/Footer}} | ||
Latest revision as of 23:56, 27 October 2010
| Home | Project Overview | Speedy reporter | Speedy switch | Speedy protein degrader | Experiments and Parts | Applications | F.A.Q | About Us |
Parts
- Ribo = Theophylline riboswitch([http://partsregistry.org/Part:BBa_K411001 BBa_K411001])
- RV = Theophylline riboswitch([http://partsregistry.org/Part:BBa_K411001 BBa_K411001]) + pSB1A2
- FRV = Theophylline riboswitch + GFP([http://partsregistry.org/Part:BBa_J04630 BBa_J04630]) + pSB1A2
- PFRV = Theophylline riboswitch + GFP([http://partsregistry.org/Part:BBa_J04630 BBa_J04630]) + pLac([http://partsregistry.org/Part:BBa_R0010 BBa_R0010]) + pSB1A2
2010.08.17
- PCR of primer&Digestion&Ligation
- Plasmid extraction
- Digestion
- PCR purification(centrifuge)
- Nanodrop
- Ligation
- Transform 20:00
2010.08.18
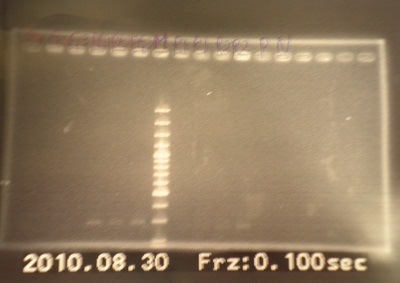
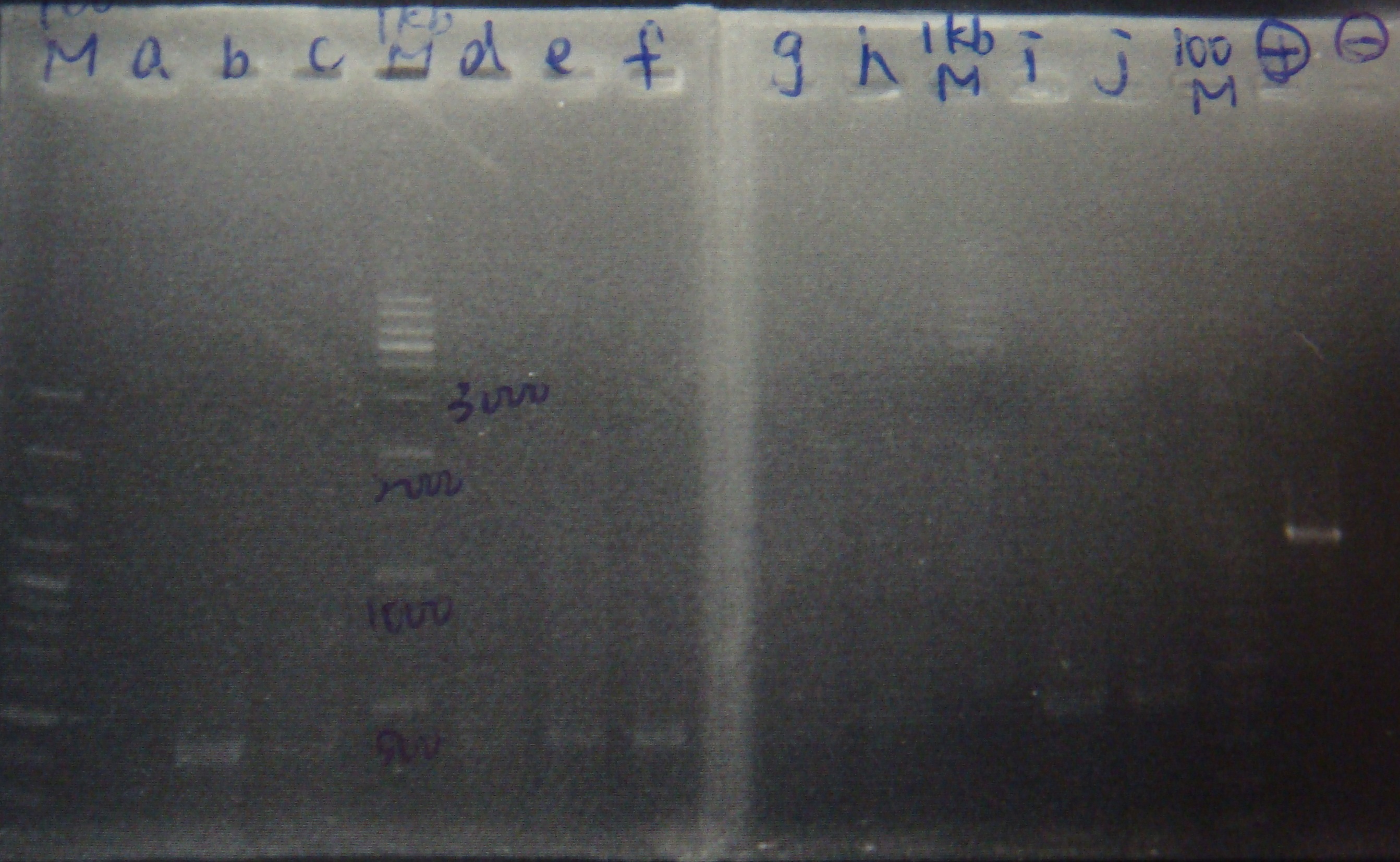
- PCR mix
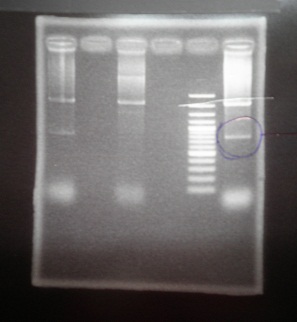
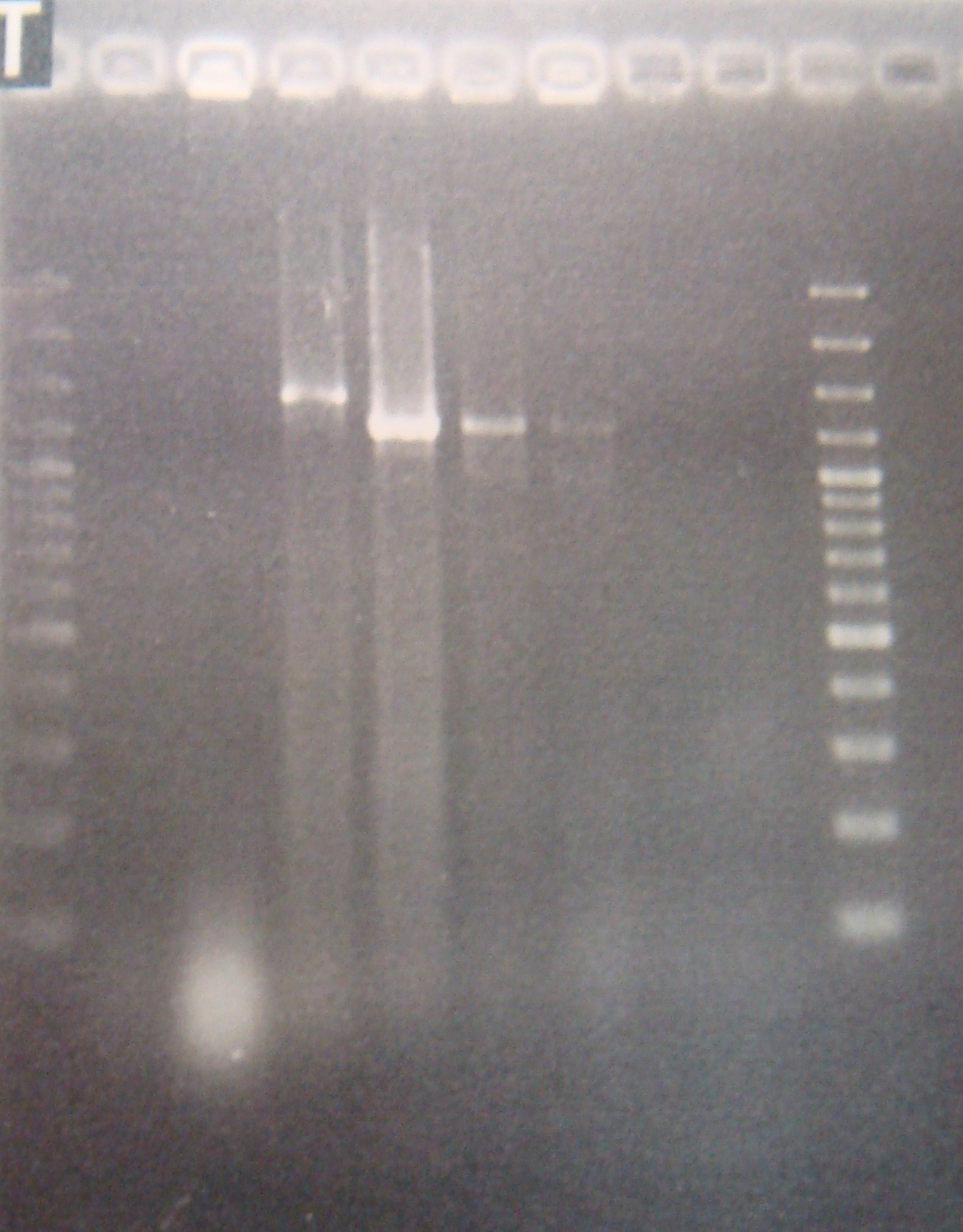
- Run gel(2%argarose 100v)
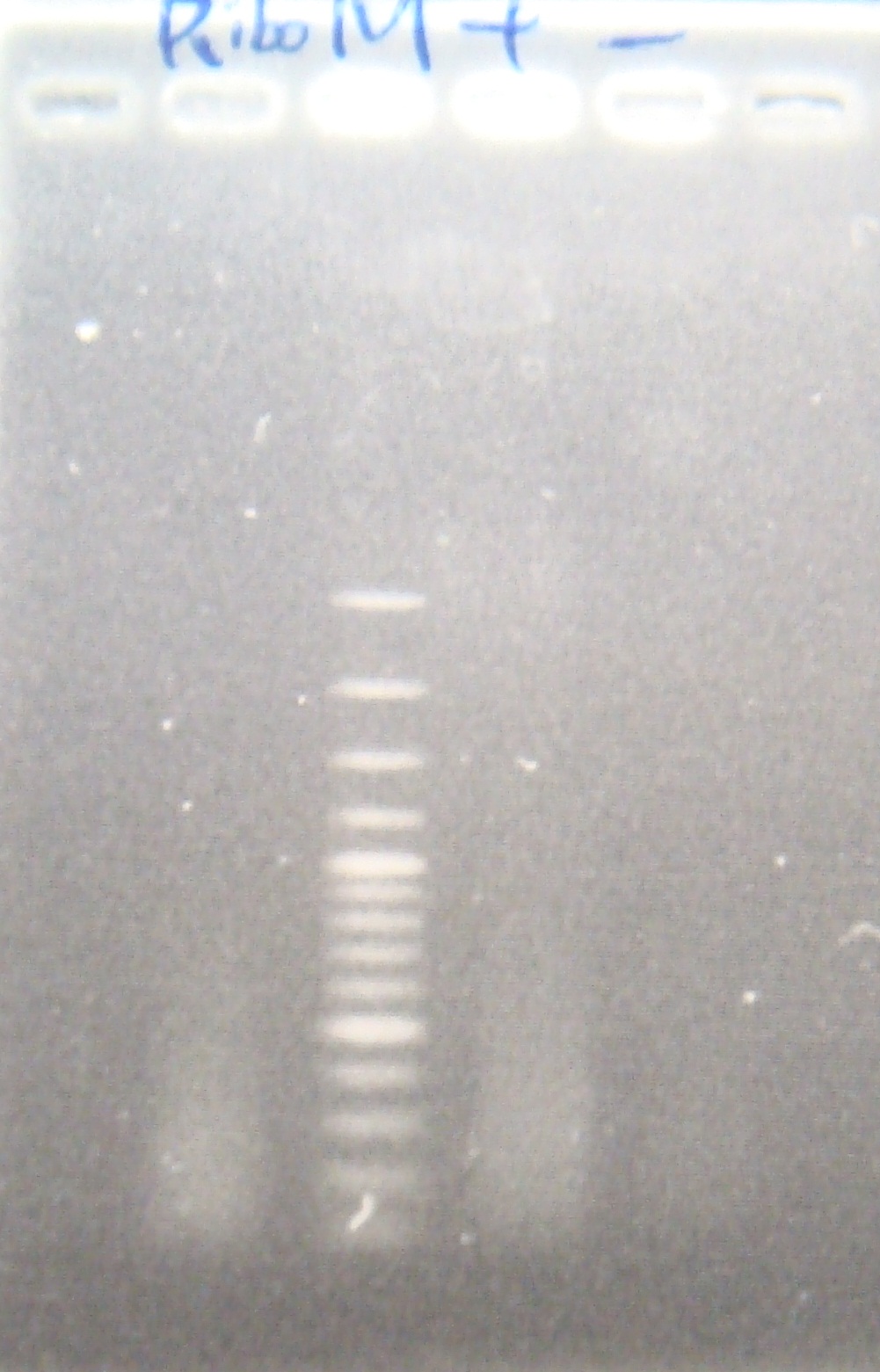
 |
|
|---|
(negative contaminated and appeared three different bands)
- 3-in-1 11:30
- Run PCR 12:00
- put Liquid culture&plate at 17:00
2010.08.19
- Because there are some mistake resulting from gel running, we decided to digest again.
- Digest 10:30
- Transformation 12:45
- At the same time, we continued plasmid extraction of the result one of yesterday.
- plasmid extraction 10:30
- Digest 11:30
- run gel 13:00
- Ligation 14:00
- Transformation 14:30
2010.08.20
- We decide to re-3-in-1 again.
- PCR mix 10:30
- 3 in 1 11:30
- Run PCR 01:00~02:30
- Run gel 15:00(100v 20 mins)
2010.08.23
2010.08.24
|
 "
"