Team:UCL London/Ethics
From 2010.igem.org
| (3 intermediate revisions not shown) | |
Latest revision as of 13:13, 27 October 2010
Synthetic Biology - 21st Century answer to our problems?
Synthetic Biology has undoubtedly opened the doorway to revolution in the scientific and engineering world! We have witnessed first hand the effect and power that can be attained behind such technology, but what about the public?
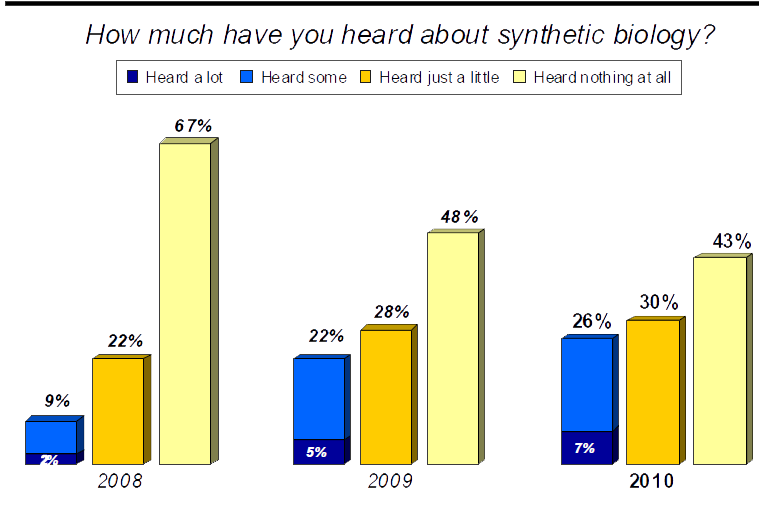
Surveys over the past few years have shown that the public awareness towards Synthetic Biology is gradually increasing, be it slowly, but nevertheless. Below is a graph from the findings of the Awareness & Impressions Of Synthetic Biology report released September 2010, and shows that slight increase in Synthetic Biology, even though the increase under each section is only in the region of around 6% since 2008.
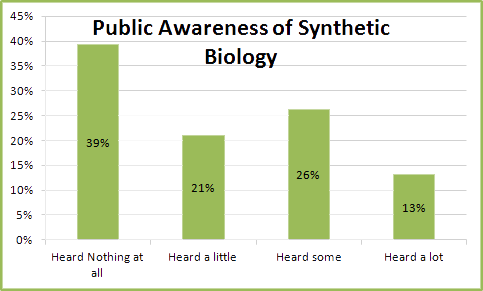
In response to this, we interviewed and asked people whether or not they had heard about Synthetic biology in all our Public Engagement events and compiled the results in the following bar chart;
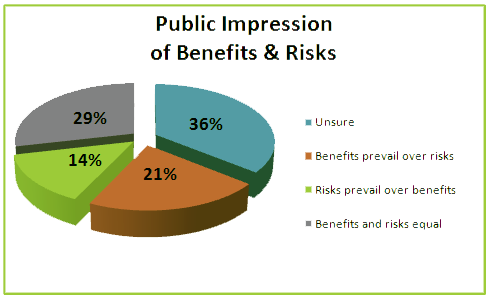
This does indeed complement the reports that public awareness of Synthetic Biology is on the rise. Here you can see that the percentage of people who have never heard of Synthetic Biology is down considerably, and more people have now been made aware of it, not necessarily indicating that they understand its purpose, but nevertheless good signs. And with initiatives like iGEM expanding globally amongst universities and institutes, we expect this trend to continue for next few years. This analysis was really simple and we wanted to go that one step further and find out what people's views of synthetic biology were and what they wanted to come of this emerging discipline. the following chart is that of people's opinions how they would balance the positives and negatives of Synthetic biology;
It was really interesting speaking and interviewing individuals because a wide range of issues and opinions were raised, from being able to implement synthetic biology techniques into the growth of livestock, to Is there a rogue element to this?.
Intellectual Property rights
Synthetic Biology is still emerging and one real issue which has been raised consistently time after time are IP rights. Solid foundations around Intellectual Property in Synthetic Biology has yet to be established and we followed this up by interviewing Lawford Davies Denoon for information on such legal issues, especially given their 25 years of experience in the legal side of life sciences which will help us understand the complexity of IP rights in the life sciences sector.
We've learnt that in order to protect our new product, some vital steps must be taken into consideration for this to become feasible. Firstly, we may have to file several patents to cover original cell line, concept, process improvements etc. It is essential that your protect yourself in terms of abiding by Trade Secrets and keeping information confidential otherwise if leaked or disclosed than the jeopardy of all your effort may be put on the line. Eventually once established, we can implement trademarks to protect our "company" as a gold standard from others in competition with us.
Bio-security is an issue which arises due to the lack of solid IP rights in Synthetic Biology, and so this openness whereby information is advertised to the world essentially does raise security issues and concerns as that information can easily be taken, manipulated and used in means other then those intended, putting the lives of people in harms way.
Regulations
Having interviewed and discussed our project with LLD
• Cells are not present in the end product. As our cells are used to improve the manufacturing process and are not used in vivo the regulatory position becomes much simpler. There is no need to perform clinical trials in order to market our product.
• It is up to manufacturers to ensure complete removal of cells/ prove cell inactivity or safety in final product. It is important to show that hypoxia cells do not contaminate the final product. This can be done by removing them (through centrifugation or membrane filtration) or if this isn’t possible then by demonstrating that the cells are inert and do not have physiological effects or by inactivating them (which will need to be proven after each batch). If the cells are present in the end product then they may require clinical trials to prove physiological inactivity unless it is analogous to an already existing product.
• Manufactured to cGMP standards. In order to maximise our potential market it is fundamental that our products are manufactured to current Good Manufacturing Practice standards. Intellectual Property We will generally be employing one of two types of applicable IP in order to protect our product.
• Patents- may have to file several to cover original cell line, concept, process improvements etc. Patents are useful as they provide a complete monopoly for the life of the patent (20 years with the potential for supplementary protection certificates- although very unlikely in this case). They are very time consuming, more so if international protection is necessary, and expensive (approximately £200,000 per patent with further costs incurred for litigation). However, if the patent is good the protection is thorough and there will be a definite period of market exclusivity where the company can establish itself as the ‘gold standard’ for this type of product until the patent expires. Can’t patent gene sequence itself but can patent it’s effects.
• Trade Secrets- Keeping the information confidential . This much cheaper alternative relies on people keeping secrets and is therefore a high risk strategy- if the information is divulged there is no protection against competitors. Saying that, this type of IP has no expiration date- it lasts as long as the secret does and is cost-free.
• Trademarks (eventually)- Once established, to protect your company as a ‘gold standard’ from other competitors. If established as a gold standard, may need to employ trademark law to protect company image.


 "
"







 Twitter
Twitter Facebook
Facebook UCL
UCL Flickr
Flickr YouTube
YouTube