Team:Wisconsin-Madison/safety
From 2010.igem.org
(→(2) Issues of Project Ramifications:) |
(→iGEM Questionnaire) |
||
| (11 intermediate revisions not shown) | |||
| Line 27: | Line 27: | ||
<div id="page_content"> | <div id="page_content"> | ||
</html> | </html> | ||
| - | == | + | ==Safety== |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
<i>"The attitudes and actions of those who work in the laboratory determine their own safety, and that of their colleagues and of the community. Laboratory equipment and design can contribute to safety only if they are used properly by people who are genuinely concerned and knowledgeable about safety issues."</i><br><br> | <i>"The attitudes and actions of those who work in the laboratory determine their own safety, and that of their colleagues and of the community. Laboratory equipment and design can contribute to safety only if they are used properly by people who are genuinely concerned and knowledgeable about safety issues."</i><br><br> | ||
<i>If scientists in this new field want to reach for the sky, they must first pull themselves from the underground, for they have the World to prove to us.</i> | <i>If scientists in this new field want to reach for the sky, they must first pull themselves from the underground, for they have the World to prove to us.</i> | ||
===Why Care About Safety?=== | ===Why Care About Safety?=== | ||
| - | <b>Synthetic Biology is a new and emerging field. Due to its nature, Synthetic Biology has high promises for greatly benefiting mankind: new cures, new treatments, new methods of fuel production, new chemical syntheses, etc. We are on a brink of a new revolution. At the same time, as the field becomes more powerful and its methods more docile, it posits incredible dangers if put into the wrong hands or if mistakes is made. As scientists in a emerging field, it is our duty to carefully evaluate any possible safety issues, not only because of general safety concerns, but also because any breach in safety has the potential for to demolish any positive public and scientific opinion. Though this new born field has great prospects, any negligence can ruin the field's reputation. Essentially, we must prove to the general public and other scientists that the potential dangers of Synthetic Biology can effectively recognized, controlled, and prevented. If we cannot do this, then we are no scientists.</b> | + | <b>Synthetic Biology is a new and emerging field. Due to its nature, Synthetic Biology has high promises for greatly benefiting mankind: new cures, new treatments, new methods of fuel production, new chemical syntheses, etc. We are on a brink of a new revolution. At the same time, as the field becomes more powerful and its methods more docile, it posits incredible dangers if put into the wrong hands or if mistakes is made. As scientists in a emerging field, it is our duty to carefully evaluate any possible safety issues, not only because of general safety concerns, but also because any breach in safety has the potential for to demolish any positive public and scientific opinion. Though this new born field has great prospects, any negligence can ruin the field's reputation. Essentially, we must prove to the general public and other scientists that the potential dangers of Synthetic Biology can effectively recognized, controlled, and prevented. If we cannot do this, then we are no scientists.</b> |
| - | + | ||
===What Safety Concerns Exist For Synthetic Biology=== | ===What Safety Concerns Exist For Synthetic Biology=== | ||
The safety considerations for this project requires extensive reflection on the latent and inherent hazards of ingesting a genetically modified bacterial strain. We wish to acknowledge and assess any possible hazards associated with a such a project. Since Synthetic Biology entails the engineering or modification of an organism for some useful purpose, we would like to examine the safety issues associated with the entire developmental process of creating a genetically engineered organism as is applicable to our iDiet project. | The safety considerations for this project requires extensive reflection on the latent and inherent hazards of ingesting a genetically modified bacterial strain. We wish to acknowledge and assess any possible hazards associated with a such a project. Since Synthetic Biology entails the engineering or modification of an organism for some useful purpose, we would like to examine the safety issues associated with the entire developmental process of creating a genetically engineered organism as is applicable to our iDiet project. | ||
Throughout the entire development process of a new organism, from initial design conception to experimental implementations to real world applications, synthetic biologists must be conscience of the possible inherent dangers of bioengineering. Things like researcher safety, global health, and even market capitalization should all be considered when designing and preparing an organism for some world application:<br> | Throughout the entire development process of a new organism, from initial design conception to experimental implementations to real world applications, synthetic biologists must be conscience of the possible inherent dangers of bioengineering. Things like researcher safety, global health, and even market capitalization should all be considered when designing and preparing an organism for some world application:<br> | ||
| - | [[Image: | + | [[Image:GoBadgers.png|800px]]<br> |
| + | |||
===What Safety Concerns Exist for iDiet?=== | ===What Safety Concerns Exist for iDiet?=== | ||
As per any scientific endeavor, assessment of both immediate and any potential ensuing safety issues is imperative prior to any direct implementations of novel scientific techniques or experiments. Further more, any engineered devices developed from these experiments require rigorous experimental study before their eventual applications outside of the laboratory. This is especially important in the field of synthetic biology, where the 'engineered device' is a genetically modified organism and the 'eventual application' involves using this organism outside of the laboratory setting and into the real world to perform some useful function. For our project, our 'engineered device' is an E.Coli modified for drug delivery; the ideal 'eventual application' involves a mammal (i.e. human) ingesting this organism so that it may release some compound (protein, drug. etc.) into the small intestine with high specificity. Therefore, we wish to separate our project's safety concerns into two groups:<br> | As per any scientific endeavor, assessment of both immediate and any potential ensuing safety issues is imperative prior to any direct implementations of novel scientific techniques or experiments. Further more, any engineered devices developed from these experiments require rigorous experimental study before their eventual applications outside of the laboratory. This is especially important in the field of synthetic biology, where the 'engineered device' is a genetically modified organism and the 'eventual application' involves using this organism outside of the laboratory setting and into the real world to perform some useful function. For our project, our 'engineered device' is an E.Coli modified for drug delivery; the ideal 'eventual application' involves a mammal (i.e. human) ingesting this organism so that it may release some compound (protein, drug. etc.) into the small intestine with high specificity. Therefore, we wish to separate our project's safety concerns into two groups:<br> | ||
| Line 65: | Line 52: | ||
====(1) The Immediate Safety Concerns:==== | ====(1) The Immediate Safety Concerns:==== | ||
<i>Immediate Safety Concerns encompasses general lab safety, strident hygiene, and proper containment of organisms used within the laboratory.</i><br><br> | <i>Immediate Safety Concerns encompasses general lab safety, strident hygiene, and proper containment of organisms used within the laboratory.</i><br><br> | ||
| - | Our project deals exclusively with Escherichia coli, which is classified as a Biosafety Level 1 organism. (Chromosomal DNA from Salmonella was used, but was obtained from a separate lab with the appropriate biosafety measures; no Salmonella was ever used in our laboratory.) Necessary general laboratory precautions were taken to ensure proper safety and to adhere to | + | Our project deals exclusively with Escherichia coli, which is classified as a Biosafety Level 1 organism. (Chromosomal DNA from Salmonella was used, but was obtained from a separate lab with the appropriate biosafety measures; no Salmonella was ever used in our laboratory.) Necessary general laboratory precautions were taken to ensure proper safety and to adhere to [http://www.cdc.gov/biosafety/ CDC] specified guidelines. Proper equipment usage and knowledge of general [http://emergency.cdc.gov/documents/PPTResponse/table3abiosafety.pdf BSL 1 safety techniques] were required for any individuals working on experiments within the lab. A safety 'mini-classes' and quizzes were also required per the [http://fpm-www3.fpm.wisc.edu/biosafety/ university policy] for undergraduate lab work. Appropriate lab attire was worn at all times (googles, gloves, lab coats) to guarantee individuals in the lab (whether performing experiments or not). Proper hygiene (of the research lab and individuals) was also exercised to achieve proper containment of any organisms cultured in the lab. |
As for project-specific experiments, acid dunking experiments were necessary for our project, therefore, knowledge of general chemistry safety was necessary for students working with highly acidic or basic solutions. Other experiments required no further safety measures other than those specified in the BSL 1 guidelines. | As for project-specific experiments, acid dunking experiments were necessary for our project, therefore, knowledge of general chemistry safety was necessary for students working with highly acidic or basic solutions. Other experiments required no further safety measures other than those specified in the BSL 1 guidelines. | ||
---- | ---- | ||
| + | |||
====(2) Issues of Project Ramifications:==== | ====(2) Issues of Project Ramifications:==== | ||
<i>Project Ramifications involves any possible imaginable hazards, including any hazards to health of an individual or a community, or any socio-economic hazards caused from the application of a bioengineered organism.</i><br><br> | <i>Project Ramifications involves any possible imaginable hazards, including any hazards to health of an individual or a community, or any socio-economic hazards caused from the application of a bioengineered organism.</i><br><br> | ||
| Line 100: | Line 88: | ||
We would like to ask the following questions related to the failures or successes of each aspect of functionality: | We would like to ask the following questions related to the failures or successes of each aspect of functionality: | ||
| - | <b>"Will the iDiet bacteria cause any harm to the individuals ingesting?"</b | + | <b>"Will the iDiet bacteria cause any harm to the individuals ingesting?"</b> |
<br> | <br> | ||
| - | <i>Enzyme Choice:</i> Would the enzyme or drug being released have potential to cause harm? This is a issue that depends on the specific application of iDiet. For this project we focused on the release of the well characterized lactase enzyme. Here we doubt that the release of this enzyme would have any serious and unexpected ill-effects on the human body. Though other enzymes or drugs could be implemented into iDiet, the specifics of the physiological reactions of the human body to that enzyme/drug would have to be explored further. | + | <i>Enzyme Choice:</i> Would the enzyme or drug being released have potential to cause harm? This is a issue that depends on the specific application of iDiet. For this project we focused on the release of the well characterized lactase enzyme. Here we doubt that the release of this enzyme would have any serious and unexpected ill-effects on the human body. Though other enzymes or drugs could be implemented into iDiet, the specifics of the physiological reactions of the human body to that enzyme/drug would have to be explored further. |
::::::::::{| border="1" | ::::::::::{| border="1" | ||
|- | |- | ||
| Line 110: | Line 98: | ||
<i>Strain Choice: </i> | <i>Strain Choice: </i> | ||
| - | The more serious issue is whether the bacteria itself is harmful to human health. For this reason, we chose E.Coli. E.Coli is, for the most part, harmless to humans. In fact, it already lives inside of the human gut. Though there are some strains that have the potential to release toxins in the human body, appropriate choice of strains can mitigate this worry. Any appropriate strain choice may be the nonpathogenic strain Nissle 1917 (O6:K5:H1), which has already been used as a [http://www.ncbi.nlm.nih.gov/pmc/articles/PMC490877/?tool=pmcentrez probiotic agent in therapeutic treatments]. | + | The more serious issue is whether the bacteria itself is harmful to human health. For this reason, we chose E.Coli. E.Coli is, for the most part, harmless to humans. In fact, it already lives inside of the human gut. Though there are some strains that have the potential to release toxins in the human body, appropriate choice of strains can mitigate this worry. Any appropriate strain choice may be the nonpathogenic strain Nissle 1917 (O6:K5:H1), which has already been used as a [http://www.ncbi.nlm.nih.gov/pmc/articles/PMC490877/?tool=pmcentrez probiotic agent in therapeutic treatments]. |
::::::::::{| border="1" | ::::::::::{| border="1" | ||
|- | |- | ||
| Line 130: | Line 118: | ||
If iDiet were ever actually implemented and become a staple in many diets, the bacteria would most certainly be released into the environment. As with anything that is engineered, things never work perfectly as expected, so we cannot assume our lysis mechanism would be absolutely complete. Any unlysed bacteria would most likely be eventually excreted. Once this happens, other mammals (mice, rats, dogs) are most likely to eventually ingest the bacteria. With enough time, the bacteria would be ingested by most warm-blooded mammals. | If iDiet were ever actually implemented and become a staple in many diets, the bacteria would most certainly be released into the environment. As with anything that is engineered, things never work perfectly as expected, so we cannot assume our lysis mechanism would be absolutely complete. Any unlysed bacteria would most likely be eventually excreted. Once this happens, other mammals (mice, rats, dogs) are most likely to eventually ingest the bacteria. With enough time, the bacteria would be ingested by most warm-blooded mammals. | ||
Whether this release would cause any environmental damage is a difficult question. We would have to know not only the physiological effects that the bacteria has on other mammals | Whether this release would cause any environmental damage is a difficult question. We would have to know not only the physiological effects that the bacteria has on other mammals | ||
| - | but also the effects of the enzyme or drug contained in the bacteria. Since this is majorly unknown, and the probability for release is so <span style="color:red">HIGH</span>, we take the safest approach and assume the worst and we evaluate this as a <span style="color:red">HIGH</span> environmental risk. | + | but also the effects of the enzyme or drug contained in the bacteria. Since this is majorly unknown, and the probability for release is so <span style="color:red">HIGH</span>, we take the safest approach and assume the worst and we evaluate this as a <span style="color:red">HIGH</span> environmental risk. |
::::::::::{| border="1" | ::::::::::{| border="1" | ||
|- | |- | ||
| Line 137: | Line 125: | ||
|}Note:<i>Evaluated as HIGH due to lack of testing</i><br> | |}Note:<i>Evaluated as HIGH due to lack of testing</i><br> | ||
| - | == | + | ==iGEM Questionnaire== |
| - | + | 1. '''Would any of your project ideas raise safety issues in terms of: researcher safety, public safety, or environmental safety?''' | |
| - | + | Please refer above to the "Safety" section. | |
| - | + | <br><br> | |
| - | + | 2. '''Do any of the new BioBrick parts (or devices) that you made this year raise any safety issues? If yes, did you document these issues in the Registry? how did you manage to handle the safety issue? How could other teams learn from your experience?''' | |
| - | + | Our part components include fluorescent proteins, digestion proteins (lactase enzyme), promoters, and genes for encapsulation through the human gut. The compositions of such part pose no threat on their own; none of the parts are toxic or would pose any unexpected risks. Thus we deem the individual iDiet parts safe for working within a non-pathogenic E.Coli in a laboratory setting that has <i>at least</i> suited for Biosafety Level 1 research.<br> | |
| - | + | However, the risk level may change when you combine these parts into a single organism. Please refer to "Safety" above for a detailed evaluation of the risks involved with combining these parts into a single E.Coli. | |
| - | + | <br><br> | |
| - | + | 3. '''Is there a local biosafety group, committee, or review board at your institution? If yes, what does your local biosafety group think about your project? If no, which specific biosafety rules or guidelines do you have to consider in your country?''' | |
| - | + | The UW-Madison Office of Biological Safety (OBS) assists all faculty and staff in observing safe biomedical laboratory practices as prescribed by the Centers for Disease Control and Prevention (CDC) and the National Institutes of Health (NIH), and endeavors to assure that research is done in secure facilities in compliance with all local, state, and federal regulations. The OBS is the administrative office of the Institutional Biosafety Committee (IBC).<br> | |
| + | The OBS fully supports the Wisconsin iGEM team under the conditions that the laboratory adhere strictly to safe biomedical laboratory practices and that each participating lab member completes a general safety classes for proper use in laboratory equipment, sterile technique, strident lab hygiene, and precautionary measures. | ||
| + | <br><br> | ||
| + | 4. '''Do you have any other ideas how to deal with safety issues that could be useful for future iGEM competitions? How could parts, devices and systems be made even safer through biosafety engineering?''' | ||
| + | Further exploration of non-leaky promoter systems is essential to dealing with safety issues associated with harmful genes (like toxic genes). The more control we have over gene expression, the greater degree of safety Synthetic Biologists can guarantee when they produce a engineered biological part; without control over gene expressions, we cannot predict the nature of the parts we are producing. One of our motivations for creating our Encryption project, was to produce a system with more control over promoters (visit the ''Projects'' page for more information.<br> | ||
| + | Furthermore, adequate documentation of each part is also essential for safety. Each part in the registry should be well documented, especially parts that pose a safety risk (toxic proteins); if we are not certain of the parts we are using, then we cannot assess appropriate risks for dealing with that part. Thus, we propose maintaining a 'zip-file standard' for all parts, which would contain files that document the progress of making that part (which may include restriction mapping, colony PCR gels, sequencing, complications, strain choice, etc.). Better documentation will always lead to better risk assessment. | ||
| + | <br> | ||
<html> | <html> | ||
<div> | <div> | ||
</html> | </html> | ||
Latest revision as of 17:49, 27 October 2010
Safety
"The attitudes and actions of those who work in the laboratory determine their own safety, and that of their colleagues and of the community. Laboratory equipment and design can contribute to safety only if they are used properly by people who are genuinely concerned and knowledgeable about safety issues."
If scientists in this new field want to reach for the sky, they must first pull themselves from the underground, for they have the World to prove to us.
Why Care About Safety?
Synthetic Biology is a new and emerging field. Due to its nature, Synthetic Biology has high promises for greatly benefiting mankind: new cures, new treatments, new methods of fuel production, new chemical syntheses, etc. We are on a brink of a new revolution. At the same time, as the field becomes more powerful and its methods more docile, it posits incredible dangers if put into the wrong hands or if mistakes is made. As scientists in a emerging field, it is our duty to carefully evaluate any possible safety issues, not only because of general safety concerns, but also because any breach in safety has the potential for to demolish any positive public and scientific opinion. Though this new born field has great prospects, any negligence can ruin the field's reputation. Essentially, we must prove to the general public and other scientists that the potential dangers of Synthetic Biology can effectively recognized, controlled, and prevented. If we cannot do this, then we are no scientists.
What Safety Concerns Exist For Synthetic Biology
The safety considerations for this project requires extensive reflection on the latent and inherent hazards of ingesting a genetically modified bacterial strain. We wish to acknowledge and assess any possible hazards associated with a such a project. Since Synthetic Biology entails the engineering or modification of an organism for some useful purpose, we would like to examine the safety issues associated with the entire developmental process of creating a genetically engineered organism as is applicable to our iDiet project.
Throughout the entire development process of a new organism, from initial design conception to experimental implementations to real world applications, synthetic biologists must be conscience of the possible inherent dangers of bioengineering. Things like researcher safety, global health, and even market capitalization should all be considered when designing and preparing an organism for some world application:
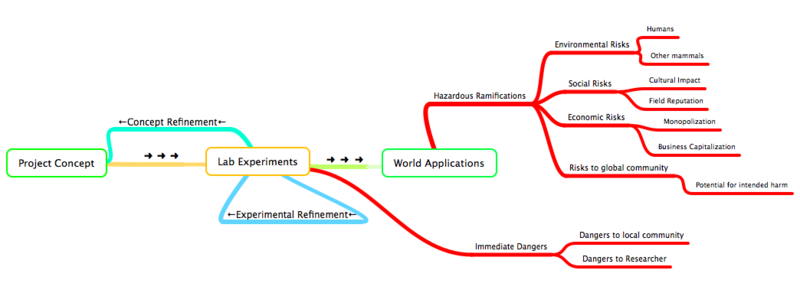
What Safety Concerns Exist for iDiet?
As per any scientific endeavor, assessment of both immediate and any potential ensuing safety issues is imperative prior to any direct implementations of novel scientific techniques or experiments. Further more, any engineered devices developed from these experiments require rigorous experimental study before their eventual applications outside of the laboratory. This is especially important in the field of synthetic biology, where the 'engineered device' is a genetically modified organism and the 'eventual application' involves using this organism outside of the laboratory setting and into the real world to perform some useful function. For our project, our 'engineered device' is an E.Coli modified for drug delivery; the ideal 'eventual application' involves a mammal (i.e. human) ingesting this organism so that it may release some compound (protein, drug. etc.) into the small intestine with high specificity. Therefore, we wish to separate our project's safety concerns into two groups:
- (1) Immediate Safety Concerns:
- a) general lab safety and general hygiene
- b) safety of experiments specific to our project
- c) safety of researchers (susceptibility of agents that we use).
- d) concerning the containment of our engineered agents within the laboratory
- a) general lab safety and general hygiene
- (2) Issues of Project Application Ramifications (eventual implementation of a drug delivery system on a mammalian subject)
- a) concerning potential probiotic and pathogenic states of an ingested genetically modified bacteria
- b) concerning the potential for unwanted release of the modified bacteria into the environment via the mammalian host.
- a) concerning potential probiotic and pathogenic states of an ingested genetically modified bacteria
(1) The Immediate Safety Concerns:
Immediate Safety Concerns encompasses general lab safety, strident hygiene, and proper containment of organisms used within the laboratory.
Our project deals exclusively with Escherichia coli, which is classified as a Biosafety Level 1 organism. (Chromosomal DNA from Salmonella was used, but was obtained from a separate lab with the appropriate biosafety measures; no Salmonella was ever used in our laboratory.) Necessary general laboratory precautions were taken to ensure proper safety and to adhere to [http://www.cdc.gov/biosafety/ CDC] specified guidelines. Proper equipment usage and knowledge of general [http://emergency.cdc.gov/documents/PPTResponse/table3abiosafety.pdf BSL 1 safety techniques] were required for any individuals working on experiments within the lab. A safety 'mini-classes' and quizzes were also required per the [http://fpm-www3.fpm.wisc.edu/biosafety/ university policy] for undergraduate lab work. Appropriate lab attire was worn at all times (googles, gloves, lab coats) to guarantee individuals in the lab (whether performing experiments or not). Proper hygiene (of the research lab and individuals) was also exercised to achieve proper containment of any organisms cultured in the lab.
As for project-specific experiments, acid dunking experiments were necessary for our project, therefore, knowledge of general chemistry safety was necessary for students working with highly acidic or basic solutions. Other experiments required no further safety measures other than those specified in the BSL 1 guidelines.
(2) Issues of Project Ramifications:
Project Ramifications involves any possible imaginable hazards, including any hazards to health of an individual or a community, or any socio-economic hazards caused from the application of a bioengineered organism.
Our iDiet project gives us a unique opportunity to explore the potential safety and ethical issues involved with the application of a genetically modified organism. We wanted to explore the potential hazardous ramifications of actually implementing bacterial delivery system into a mammalian host. Hazardous ramifications will be evaluated by the following by a calculation of the Risk involved, which is some function of Hazard and Probability:
- Risk(Hazard,Probability) = max(Hazards) x Probability
Where max(Hazard) is the degree for potential damage in the worst case scenario and Probability is the chances of that potential damage occurring. In all evaluations of risks, we will take the 'worst scenario' approach. For example, if the Hazard is High and the Probability is Unknown, then we will assume a High Risk. This way, we will always overestimate the risk.
Evaluating Risks Probability Hazard LOW -- it is not likely to happen LOW -- the potential damage is
almost non-existentMED -- it may happen under
certain circumstancesMED -- examples: non-life threatening
illness, unwanted symptoms, etc.HIGH -- it most likely will happen HIGH -- examples: fatal illness,
serious symptoms, etc.
As an overview, our drug delivery system, iDiet, provides five aspects of functionality:
- i) ingestion of bacteria (incorporation into human diet)
- ii) enzyme production
- iii) encapsulation (protection from the harsh acidic environment of the stomach)
- iv) lysis and release (releasing enzyme or drug into small intestines)
- v) effects of drug or enzyme on human
We would like to ask the following questions related to the failures or successes of each aspect of functionality:
"Will the iDiet bacteria cause any harm to the individuals ingesting?"
Enzyme Choice: Would the enzyme or drug being released have potential to cause harm? This is a issue that depends on the specific application of iDiet. For this project we focused on the release of the well characterized lactase enzyme. Here we doubt that the release of this enzyme would have any serious and unexpected ill-effects on the human body. Though other enzymes or drugs could be implemented into iDiet, the specifics of the physiological reactions of the human body to that enzyme/drug would have to be explored further.
Note: depending on enzyme choice, the risk could varyHazard: LOW Probability: LOW Risk:LOW**
Strain Choice: The more serious issue is whether the bacteria itself is harmful to human health. For this reason, we chose E.Coli. E.Coli is, for the most part, harmless to humans. In fact, it already lives inside of the human gut. Though there are some strains that have the potential to release toxins in the human body, appropriate choice of strains can mitigate this worry. Any appropriate strain choice may be the nonpathogenic strain Nissle 1917 (O6:K5:H1), which has already been used as a [http://www.ncbi.nlm.nih.gov/pmc/articles/PMC490877/?tool=pmcentrez probiotic agent in therapeutic treatments].
Note: depending on strain choice, the risk could varyHazard: MED Probability: LOW RISK: MED**
"Does the iDiet bacteria have the possibility to displace the naturally occurring bacteria in the human body?"
Strain Choice: This displacement of natural bacteria is a serious issue for iDiet. If there is even a slight hazard or safety concern associated with the iDiet bacteria (see above), this hazard will be amplified astronomically due to the overwhelming presence of the bacteria. Further more, even if the bacteria had no harmful effects, there is a risk that a change in the physiological state in the human body can wipe out the entire homogenous bacterial population. Though it has been shown that humans can survive [http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6T75-4F9JB4T-1&_user=10&_coverDate=06%2F15%2F2005&_rdoc=1&_fmt=high&_orig=search&_origin=search&_sort=d&_docanchor=&view=c&_acct=C000050221&_version=1&_urlVersion=0&_userid=10&md5=4a8a30fdd95fd0670d45a4b4525a716f&searchtype=a without gut bacteria], the presence of bacteria provides many beneficial effects.
But is this displacement probable? If lysis fails or is incomplete and the bacteria survives inside the human gut, we must ask the question of whether the bacteria has the potential to displace naturally occurring bacteria; this would most likely be dependent on the strain choice. The number bacteria cells out number the amount of human cells 10:1 in a person amounting to 10^14 cells, E.Coli being one of the most prevalent. With such HIGH number of naturally occurring bacteria, it seems unlikely that iDiet bacteria would ever become the most prevalent strain of E.Coli in the human body, nonetheless, entirely displace naturally occurring strains. However, if iDiet were to be actually implemented, it would most likely be incorporated into a human diet, where the patient is repeatably ingesting the iDiet bacteria, therefor this probability for hazard is greater than expected. However, due to the diversity of the gut flora, this probability is still LOW.
Hazard: MED Probability: LOW RISK: MED
"What potential does the bacteria have for being released into the environment and would this release cause any environmental damage?"
If iDiet were ever actually implemented and become a staple in many diets, the bacteria would most certainly be released into the environment. As with anything that is engineered, things never work perfectly as expected, so we cannot assume our lysis mechanism would be absolutely complete. Any unlysed bacteria would most likely be eventually excreted. Once this happens, other mammals (mice, rats, dogs) are most likely to eventually ingest the bacteria. With enough time, the bacteria would be ingested by most warm-blooded mammals.
Whether this release would cause any environmental damage is a difficult question. We would have to know not only the physiological effects that the bacteria has on other mammals
but also the effects of the enzyme or drug contained in the bacteria. Since this is majorly unknown, and the probability for release is so HIGH, we take the safest approach and assume the worst and we evaluate this as a HIGH environmental risk.
Note:Evaluated as HIGH due to lack of testingHazard: UNKNOWN Probability: HIGH RISK: HIGH
iGEM Questionnaire
1. Would any of your project ideas raise safety issues in terms of: researcher safety, public safety, or environmental safety?
Please refer above to the "Safety" section.
2. Do any of the new BioBrick parts (or devices) that you made this year raise any safety issues? If yes, did you document these issues in the Registry? how did you manage to handle the safety issue? How could other teams learn from your experience?
Our part components include fluorescent proteins, digestion proteins (lactase enzyme), promoters, and genes for encapsulation through the human gut. The compositions of such part pose no threat on their own; none of the parts are toxic or would pose any unexpected risks. Thus we deem the individual iDiet parts safe for working within a non-pathogenic E.Coli in a laboratory setting that has at least suited for Biosafety Level 1 research.
However, the risk level may change when you combine these parts into a single organism. Please refer to "Safety" above for a detailed evaluation of the risks involved with combining these parts into a single E.Coli.
3. Is there a local biosafety group, committee, or review board at your institution? If yes, what does your local biosafety group think about your project? If no, which specific biosafety rules or guidelines do you have to consider in your country?
The UW-Madison Office of Biological Safety (OBS) assists all faculty and staff in observing safe biomedical laboratory practices as prescribed by the Centers for Disease Control and Prevention (CDC) and the National Institutes of Health (NIH), and endeavors to assure that research is done in secure facilities in compliance with all local, state, and federal regulations. The OBS is the administrative office of the Institutional Biosafety Committee (IBC).
The OBS fully supports the Wisconsin iGEM team under the conditions that the laboratory adhere strictly to safe biomedical laboratory practices and that each participating lab member completes a general safety classes for proper use in laboratory equipment, sterile technique, strident lab hygiene, and precautionary measures.
4. Do you have any other ideas how to deal with safety issues that could be useful for future iGEM competitions? How could parts, devices and systems be made even safer through biosafety engineering?
Further exploration of non-leaky promoter systems is essential to dealing with safety issues associated with harmful genes (like toxic genes). The more control we have over gene expression, the greater degree of safety Synthetic Biologists can guarantee when they produce a engineered biological part; without control over gene expressions, we cannot predict the nature of the parts we are producing. One of our motivations for creating our Encryption project, was to produce a system with more control over promoters (visit the Projects page for more information.
Furthermore, adequate documentation of each part is also essential for safety. Each part in the registry should be well documented, especially parts that pose a safety risk (toxic proteins); if we are not certain of the parts we are using, then we cannot assess appropriate risks for dealing with that part. Thus, we propose maintaining a 'zip-file standard' for all parts, which would contain files that document the progress of making that part (which may include restriction mapping, colony PCR gels, sequencing, complications, strain choice, etc.). Better documentation will always lead to better risk assessment.
 "
"